About
RSVpreF3
-
GSK plc
-
Single-dose, 0.5-mL intramuscular injection for active immunization to prevent LRTD caused by RSV in individuals ≥60 years old
-
12 per 1,000: RSV maternal and pediatric-eligible population in the U.S.

Respiratory syncytial virus (RSV) infections continue to be a public health concern, particularly for infants and older adults (65 years and older). A common upper respiratory infection that can result in hospitalizations in severe cases, RSV infection tends to be seasonal and present with symptoms similar to those of influenza and COVID-19. The occurrence of all three infectious diseases at the same time of year contributes to what public health experts call a “triple-demic,” with an associated increase in healthcare burden particularly for the most vulnerable groups. The first approvals of RSV vaccines (RSVpreF and RSVpreF3) targeted at infants and older adults mark a significant public health milestone.

The phase 3 results for both vaccines validate the clinical efficacy of vaccines based on the RSV F protein, which was a ground-breaking discovery that accelerated the recent development of vaccines against RSV. In addition, these results bode well for the phase 3 trial results of other assets in late-stage development, which will help support the public health initiative to reduce the RSV-related disease burden.
Preliminary results from the phase 3 clinical trial evaluating AREXVY for adults aged 50 to 59 years were published in October 2023, showing that the vaccine elicited an immune response in that study population that was non-inferior to that observed in adults aged 60 years and older.
February 2020
For the prevention of LRTD caused by RSV in individuals 60 years of age and older
May 2023
June 2023
July 2023
August 2023
September 2023
Actual and expected launch:
How will RSVpreF3 impact the RSV market?
What gaps in treatment does RSVpreF3 fill?
The search for an effective vaccine against RSV, a common, contagious virus, began in the mid-1960s but remained unsuccessful until the 2010s when focus shifted to the RSV F protein. Seasonal hospitalizations due to serious respiratory illness caused by RSV continue to be a public health issue, particularly for infants, young children, older adults and individuals with underlying health conditions such as COPD and asthma. The approvals of RSV vaccines address a significant need for infectious disease control, reduced morbidity and mortality, and decreased hospital burden at times when other infectious disease outbreaks are prevalent.
What hurdles might it need to overcome to reach blockbuster status?
Although these two vaccines were the first approved, other vaccines are in late-stage development, and Beyfortus has also been approved for use in this setting. In addition to this competition, the lag in insurance coverage in the United States during the first season in which the vaccines are available and lingering vaccine hesitancy from the COVID-19 pandemic could limit uptake.

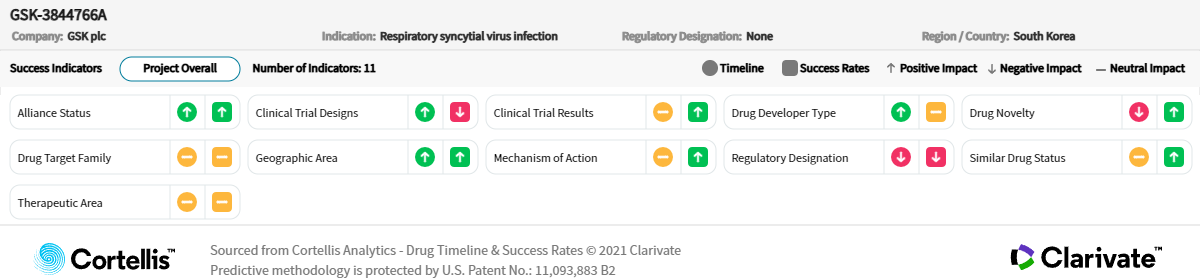
– Source: Cortellis Competitive Intelligence, Drug Timeline & Success Rates Prediction current as of November 3, 2023
Access global intelligence, advanced analytics and global experts from Clarivate.