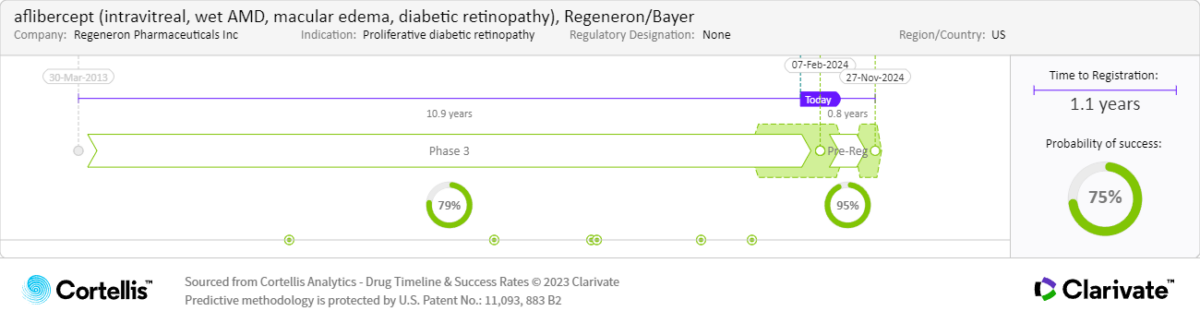
About
aflibercept
-
Bayer and Regeneron Pharmaceuticals Inc
-
VEGF inhibitor
-
Intravitreal (IVT) administration to treat wet AMD, DME and DR
-
Also being studied to treat macular edema secondary to retinal vein occlusion (RVO) as well as macular telangiectasia type 1
-
2.55M people in the G7 markets expected to be drug-treated for wet AMD by 2032
-
2.0M people with DME in the G7 markets expected to be drug-treated by 2032
-
~130K people with severe non-proliferative DR and proliferative DR in the G7 countries expected to receive drug treatment by 2032