January 2019
For patients with SCD
- Fast Track designation: U.S. FDA
April 2019
For patients with transfusion-dependent beta-thalassemia
- Fast Track designation: U.S. FDA
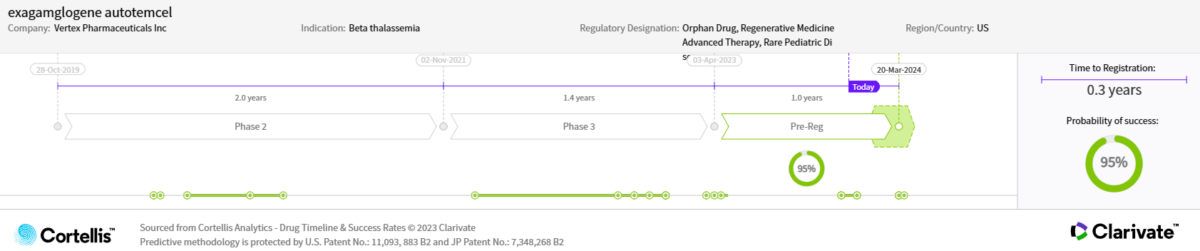
October 2019
For patients with transfusion-dependent beta-thalassemia
January 2020
For patients with SCD
April 2020
For patients with transfusion-dependent beta-thalassemia
- Orphan drug status: U.S. FDA
May 2020
For patients with SCD
- Orphan drug status: U.S. FDA
For patients with transfusion-dependent beta-thalassemia
- Regenerative medicine advanced therapy (RMAT) designation: U.S. FDA
September 2020
For patients with SCD
- Rare pediatric disease designation: U.S. FDA
October 2020
For patients with SCD or transfusion-dependent beta-thalassemia
April 2021
For patients with transfusion-dependent beta-thalassemia
January 2023
For patients with SCD
- MAAs validated: EMA and U.K. MHRA
April 2023
For patients with SCD
For patients with transfusion-dependent beta-thalassemia
August 2023
For patients with SCD
- Priority review granted: U.S. FDA
October 2023
For patients ≥12 years old with SCD with recurrent VOCs or transfusion-dependent beta-thalassemia for whom a human leukocyte antigen-matched related hematopoietic stem cell donor is not available
December 2023
For patients ≥12 years old with SCD with recurrent VOCs
March 30, 2024
For patients with transfusion-dependent beta-thalassemia
Actual and expected launch:
For patients with SCD or transfusion-dependent beta-thalassemia
- 2023: United Kingdom, United States
- 2024: European Union
Patents estimated to expire beginning in 2033