
About CALQUENCE®
-
Patented by AstraZeneca
-
Inhibitor of Bruton’s tyrosine kinase (BTK) with potential antineoplastic activity to treat CLL, SLL and previously treated MCL

How many ANDA filers with paragraph IV certification will file later this year?


U.S. market share

Source: Cortellis Generics Intelligence

Approximately 70% of MCL cases are diagnosed with advanced disease, and most patients receive treatment regardless of disease stage. Because of the aggressive nature of MCL, few patients experience an extremely durable remission, while most patients will require second or third-line treatment.
Although patients with CLL or SLL do not necessarily receive pharmacologic treatment initially, 75% of patients eventually require drug treatment.

Based on Cortellis data, there are few confirmed sources of API, although there are companies in India (all with an “Established” Corporate API Rating*) developing on the API: MSN Laboratories Private Limited, NATCO Pharma Limited, Sun Pharmaceutical Industries Ltd. and Sionc Pharmaceuticals Pvt.Ltd.Although there is minimal API manufacturing, Cortellis Generics Intelligence data show a variety of patents on manufacturing process, intermediates and product derivatives from the following companies indicating numerous generic companies evaluating the product:
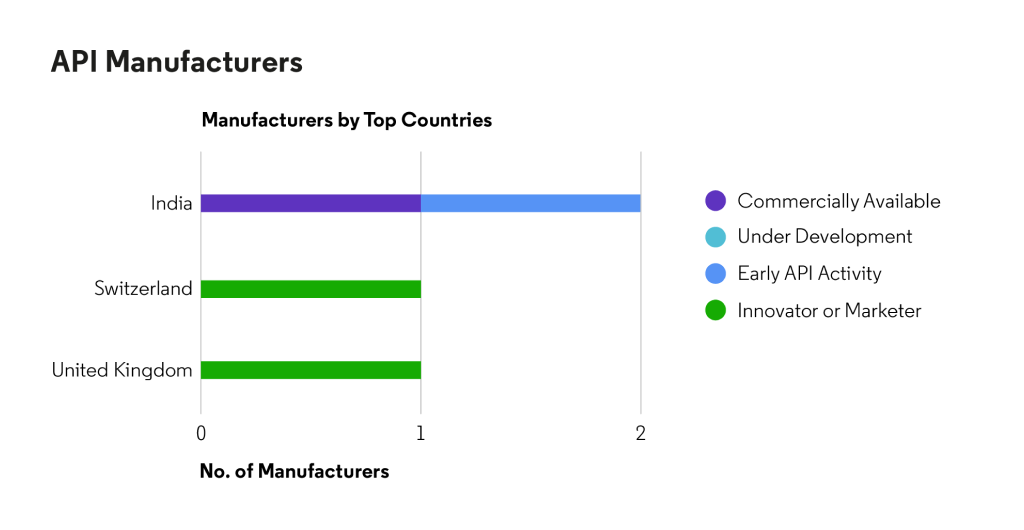
Source: Cortellis Generics Intelligence
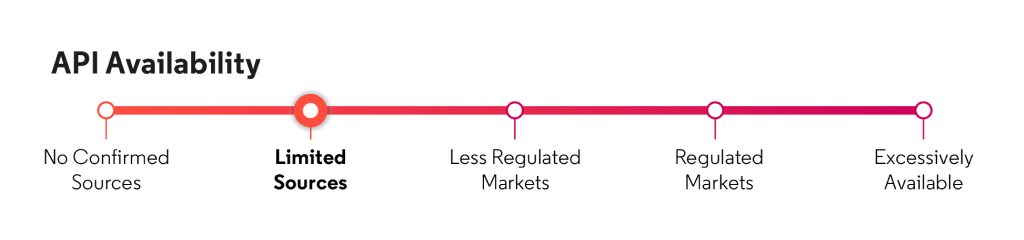
*Corporate API Rating: proprietary Cortellis Generics Intelligence analytic that indicates how capable the corporate group is of supplying bulk to regulated markets like Europe and North America.
Source: Cortellis Generics Intelligence
Data current as of April 21, 2021
Access our global intelligence, advanced analytics and global team of experts.