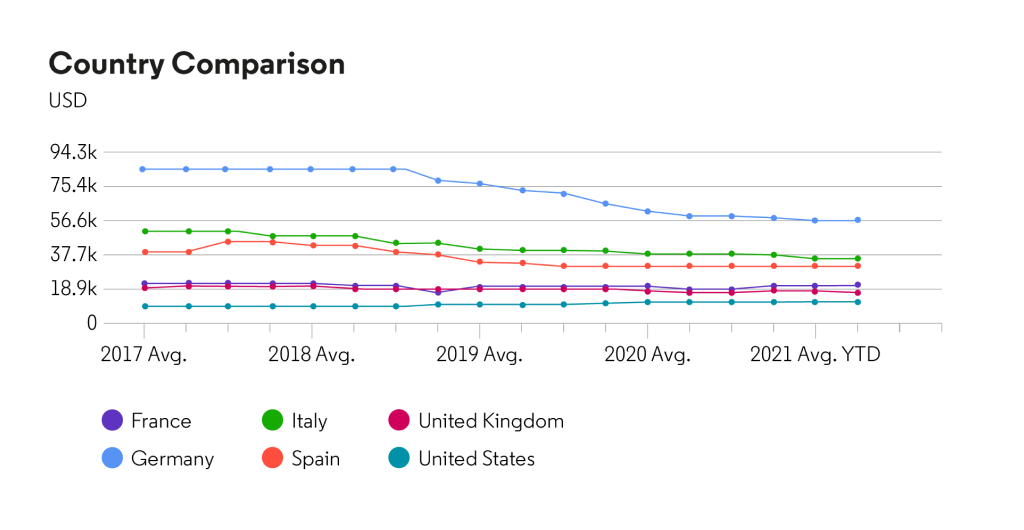
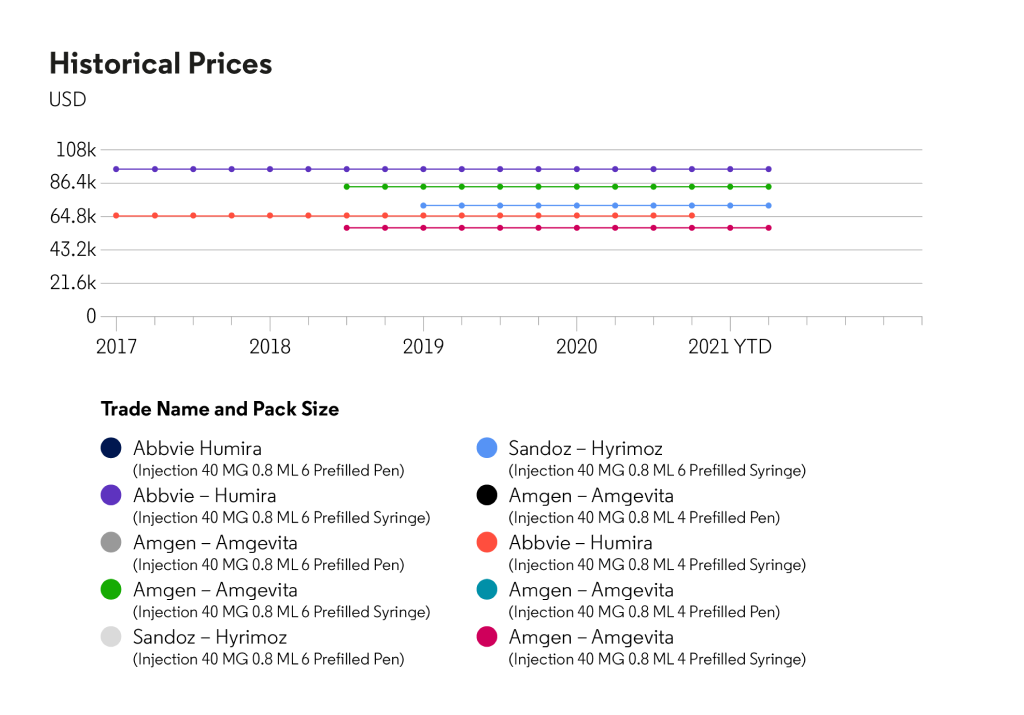
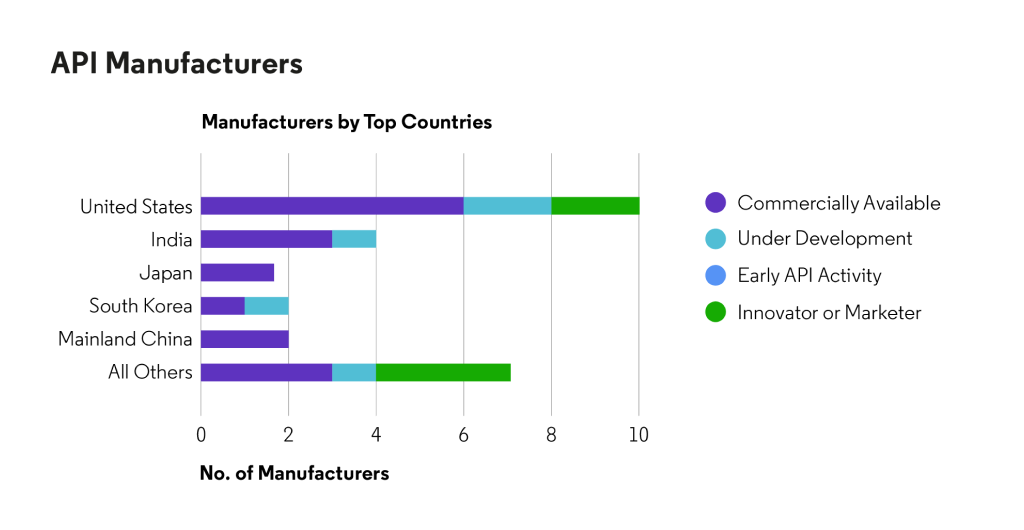
About HUMIRA®
-
Patented by AbbVie Inc.
-
TNF-α inhibitor to treat many autoimmune conditions with inflammation as a central mechanism, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, plaque psoriasis, juvenile idiopathic arthritis, ulcerative colitis, moderate to severe hidradenitis suppurativa and more