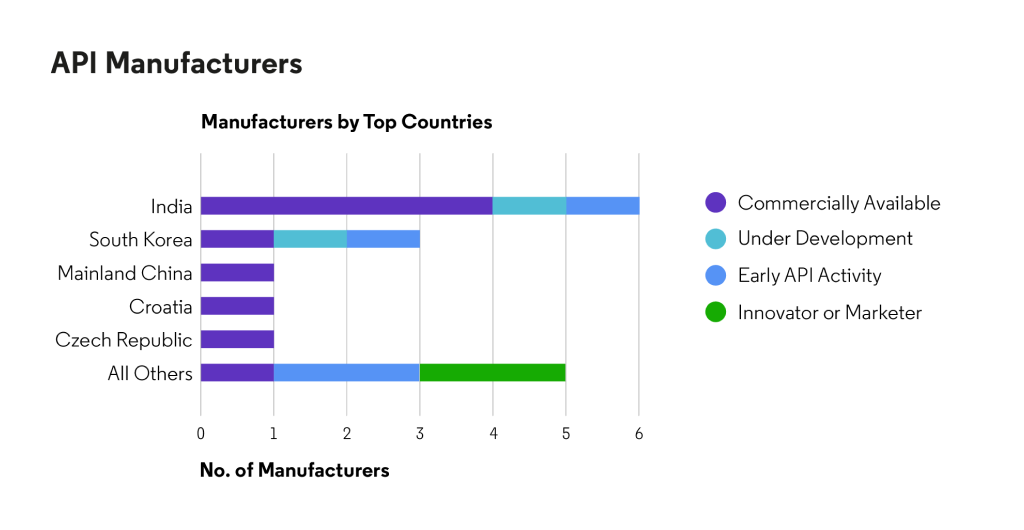
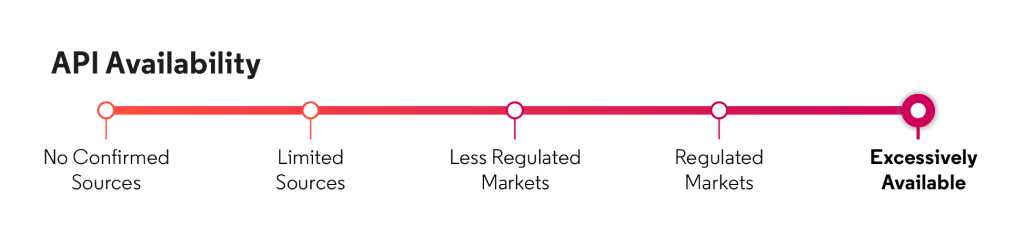
Respiratory - chronic obstructive pulmonary disease
About TASIGNA®
-
Marketed by Novartis Pharmaceuticals Corporation
-
Orally bioavailable aminopyrimidine-derivative Bcr-Abl tyrosine kinase inhibitor
-
Treatment of adult and pediatric patients with newly diagnosed or treatment-resistant Philadelphia chromosome–positive chronic myeloid leukemia (Ph+ CML)