Respiratory - chronic obstructive pulmonary disease
About HALAVEN®
-
Marketed by Eisai Inc.
-
Microtubule dynamics inhibitor
-
Treatment of refractory, metastatic breast cancer and liposarcoma



API availability
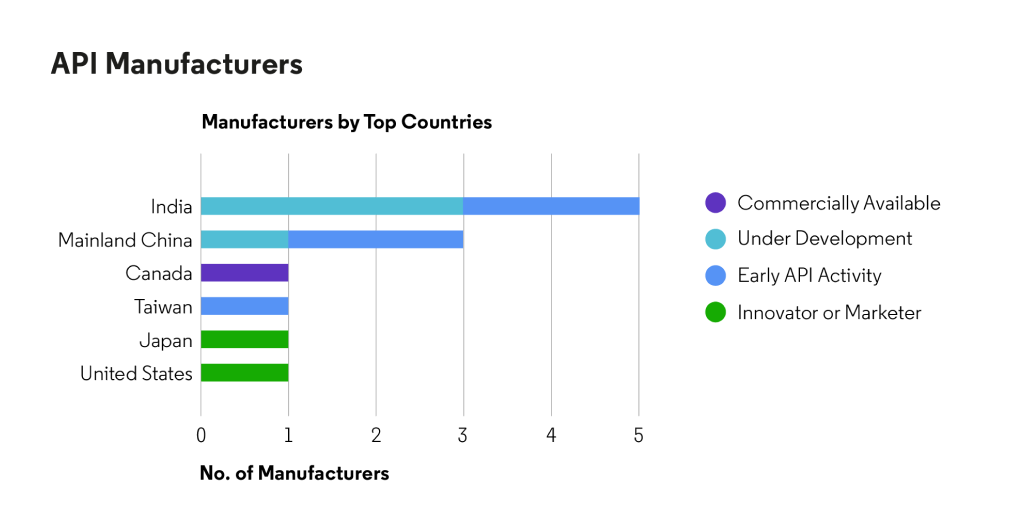
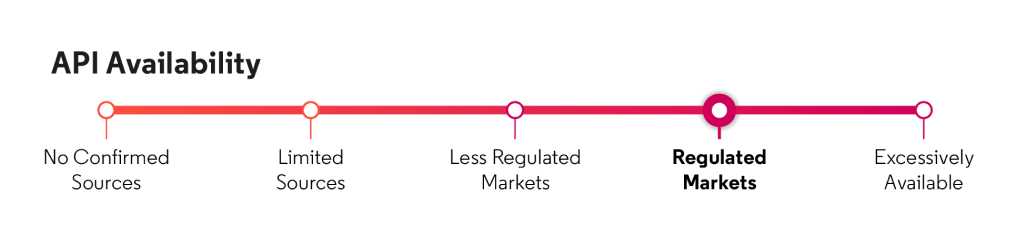
Source: Cortellis Generics Intelligence
Access our global intelligence, advanced analytics and global team of experts.