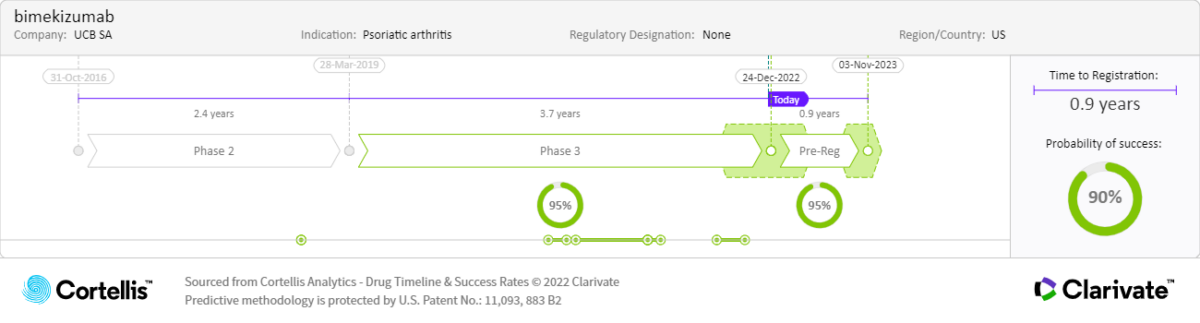
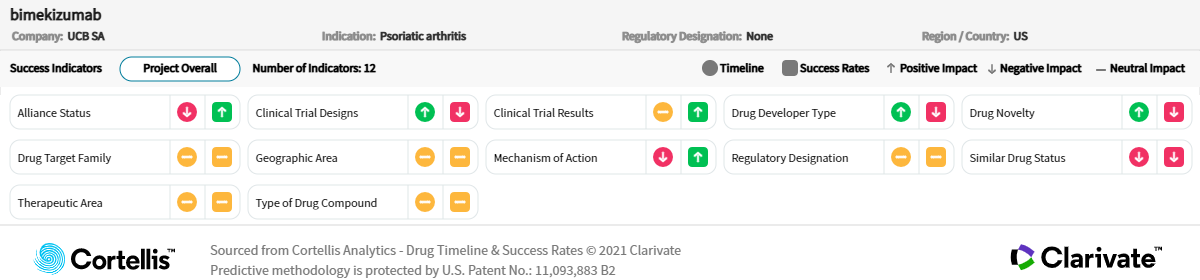
About bimekizumab
-
UCB
-
Humanized IgG1 monoclonal antibody (mAb)
-
Subcutaneous injection every four weeks for the first five treatments, then every eight weeks to treat moderate to severe plaque psoriasis
-
Also being studied to treat axial spondyloarthritis, psoriatic arthritis and hidradenitis suppurativa
-
~11.7 million symptomatic psoriasis cases in the G7 markets in 2021
-
80-90% of patients with psoriasis have plaque psoriasis