Real World Evidence (RWE) analysis dashboard
The RWE dashboard supports the validation and analysis of potential pharmacovigilance signals. It provides a unique translational view of drugs and targets, integrating preclinical data, clinical development and pharmacovigilance insights, and is fully integrated with more than 1.4 million curated alerts and other OFF-X functionalities and analytics.
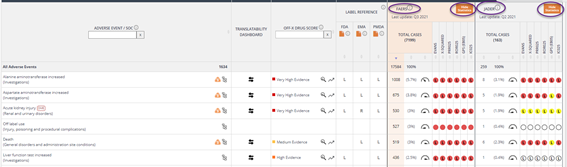
The RWE dashboard includes curated reports from the FDA adverse event reporting system (FAERS) and the Japanese Adverse Drug Reaction (JADER) databases.
Within the dashboard, you can see:
- Total number of cases and events (a case may contain multiple events)
- Number of reports for each specific adverse event. Percentage of the total number of reports is also provided
- The speedometer indicates the growth of new reports compared to those added the previous quarter
- Statistics: Show or hide the statistics as desired. The following icons and notations are used to visualize the statistical scores:
- Red: Adverse events with at least 3 reports available and for which there is a potential statistical associations (does not imply causality)
- Yellow: Adverse Events with at least 3 reports available and for which it appears to be no statistical association
- White: Adverse Events with fewer than 3 records. Statistical association not computed
- “L” inside a circle indicates that the Adverse Event has been included in the drug label, approval document or document describing changes since the drug’s authorization by a regulatory agency (FDA, EMA or PMDA).
- “R” inside a circle indicates that at least one regulatory agency (FDA, EMA, PMDA) is reviewing or has reviewed a given event (and no further action was deemed necessary)
- Purple border: indicates that reports older than 5 years have been included
- 1st: indicates that a drug -event relationship has been mentioned in a case safety report for the first time in the last quarter
Find further information in this user guide.