OFF-X Translational Safety Intelligence
Unique insights into drug and class toxicity using integrated preclinical and clinical data
OFF-X de-risks drug development and pharmacovigilance
Safety issues are a leading cause of costly failures and adverse reactions. OFF-X helps pharma and biotech anticipate and mitigate these risks by consolidating clinical and preclinical safety data into a single platform for proactive decision making.
Safer drugs, from bench to market
OFF-X helps you monitor toxicology and safety signals, mitigate liabilities and de-risk assets across the entire drug lifecycle, from discovery to post-market surveillance.
Tap into unique drug safety data coverage
Access integrated preclinical and clinical safety data, including early-stage toxicity, clinical adverse events and PV data, for comparative safety profiling, signal prioritization and pathway-based risk analysis.
Gain confidence in every decision
Rely on expertly curated, high-quality translational safety intelligence—backed by leading subject matter experts—to make informed choices with assurance.
A translational safety solution for proactive risk management

Your safety radar, always on
Get instant alerts on safety signals and new data so you can act fast, reduce risk, and keep development on track.

Comprehensive data sources
Draw insights from peer-reviewed journals, congresses, trial registries, and global regulatory agency communications and approval packages.

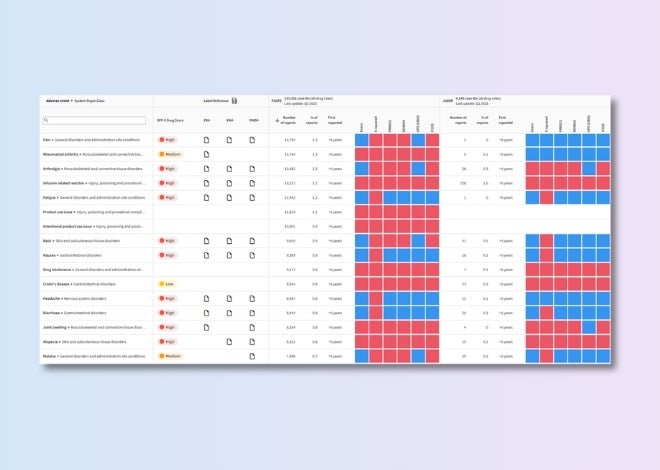
Drug–adverse event evidence analytics
Prioritize and validate safety signals using transparent, evidence-based scores for each drug–adverse event association.

Target class safety risk score
Identify emerging liabilities across target classes using a scoring system that highlights patterns, flags trends, and guides mitigation efforts.

Interactive translational pathways
Assess and prioritize investigational target safety via pathway maps that trace signaling cascades to reveal mechanisms and risks.

Spot safety risks faster
Visualize safety insights for novel drugs and combinations with side by side comparisons to anticipate liabilities and explain toxicities.
Accelerate outcomes with preclinical and clinical safety analytics
Uncover safety liabilities early
Identify safety risks early by evaluating targets in their pathway context and monitoring how new candidates translate from preclinical to clinical.

Minimize costly clinical trial delays
Spot emerging adverse events, benchmark class profiles, and analyze unexpected findings quickly to design safer, more resilient clinical trials.

Prioritize and validate PV signals faster
Quickly prioritize and validate PV signals with classbased evidence, improving regulatory readiness and supporting confident safety monitoring.
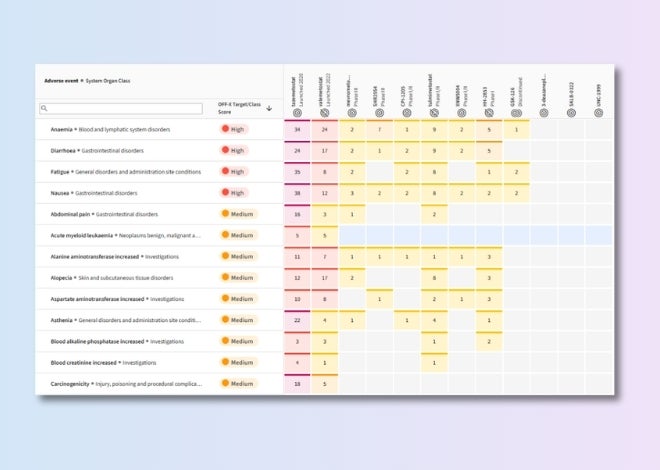
Benchmark safety profiles
Track competitors’ development progress and assess the latest target safety assessments to mitigate risks and inform go/no-go decisions.
Frequently asked questions
Find answers to common questions about our translational drug safety analytics, from data sources and coverage to key benefits.
OFF-X is a translational safety intelligence platform that helps pharma and biotech companies anticipate and monitor safety liabilities, benchmark drug and target safety profiles, and detect adverse events for compounds at every stage of drug development and post-approval.
Unlike other safety intelligence providers, OFF-X collects and curates data from a wide range of sources, integrating preclinical toxicity data, clinical adverse event data and pharmacovigilance data in a single platform. Additionally, OFF-X covers drugs across all stages of the lifecycle, including approved, unapproved and discontinued.
OFF-X helps drug developers anticipate the toxicity profile of new targets and drug candidates, and monitor their human translation. OFF-X also supports dynamic target safety assessments by pre-empting the safety of novel targets based on up/downstream targets in their signaling pathways.
With OFF-X, clinical teams gain actionable insights to de-risk studies and maximize the chances of clinical success by comparing same-in-class drugs as they advance in development. Our translational safety intelligence helps clinical researchers understand how their drug’s safety profile is influenced by patient characteristics (such as condition, age and gender) and can help them define the right clinical trial endpoints and select the most suitable safety biomarkers.
In the event of unexpected safety findings, OFF-X empowers researchers to act quickly by helping them understand these findings and support effective mitigation strategies.
OFF-X empowers PV teams to easily prioritize safety signals and anticipate potential new signals based on members of the same class, and monitor the role of concomitant medications in unexpected adverse reactions. Pharmacovigilance professionals can anticipate risk factors by benchmarking the safety profiles of investigational, launched and discontinued drugs by target classes. In addition, they can easily compile publications for aggregated reports and prepare comprehensive responses to health authorities’ requests.
Updated daily, OFF-X covers drugs and targets in all phases of R&D & post-marketing, and all drug modalities. A team of experts curates the content from a wide range of sources, including:
- Peer-reviewed literature
- Congresses / conference proceedings
- Clinical Trial registries
- Real World Evidence Databases like FAERS & JADER
- Regulatory documents
- Company communications
- Proprietary disease pathway maps
Yes, you can leverage bulk preclinical and clinical data from this translational safety platform as a data feed or API to populate internal data and analytics solutions.
Improve drug safety decisions
Contact us to schedule a demo of OFF-X
"The FDA will continue using OFF-X as a solution to identify potential adverse events associated with molecular targets and new and marketed drugs and its utility in the regulatory review process"

Food and Drug Administration (FDA) and Clarivate Extend Material Transfer Agreement for Three Additional Years
Partnership enables agency-wide access to OFF-X translational safety intelligence solution London, U.K., May 23, 2023 –– Clarivate Plc (NYSE: CLVT), a global leader in connecting people and organizations to intelligence they…
Drive successful drug development with preclinical and clinical safety data
3m+
6k+
14k+
15k+
16m+
19k+
Resources
Fraud Warning
Please be advised that recently there have been fraudulent job offers and interviews using the Clarivate name, logo and even names of our colleagues.
Please be aware that Clarivate will:
- Never ask for payment of any kind as part of our hiring or onboarding processes
- Never ask an applicant to email sensitive personal information, such as a Social Security Number, birthdate, credit card or bank account information
- Never issue pre-employment checks to purchase office supplies
- Never ask you to pay up for an external course and upskill
If you have any question about a position posted in our company name, please check our current open positions on the Clarivate website Careers pages or contact one of our recruiting team members directly.
If you have been the victim of a scam, please contact your local law enforcement agency.
Federal Transparency In Coverage Rule
This link leads to the machine-readable files that are made available in response to the federal Transparency in Coverage Rule and includes negotiated service rates and out-of-network allowed amounts between health plans and healthcare providers. The machine-readable files are formatted to allow researchers, regulators, and application developers to more easily access and analyze data.