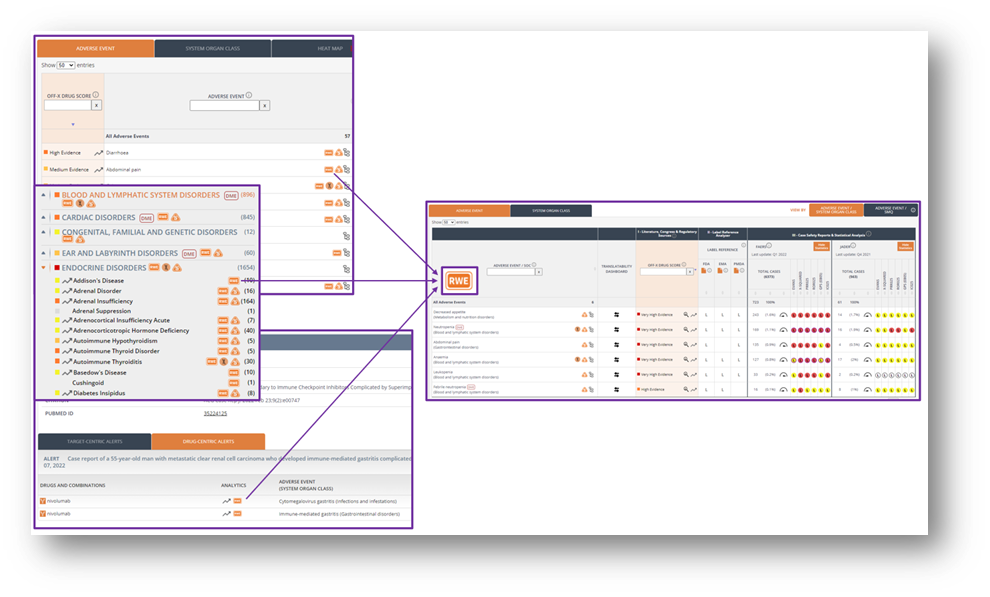
In order to further integrate the OFF-X Real-World Evidence Analysis Dashboard in pharmacovigilance workflows, links to this tool have been added to reference records and drug record’s master view and safety alerts. The RWE Analysis Dashboard offers a unique, integrated view of curated data from multiple data sources (biomedical literature, company communications, clinical trial registries), regulatory safety intelligence and individual case safety reports (ICSRs) from FAERS and JADER. Use these new links as a shortcut to easily assess the significance of the real-world data associated with safety events reported elsewhere. Whenever ICSRs are available in FAERS/JADER for a drug-adverse event pair, a shortcut to the RWE Analysis Dashboard, filtered by your adverse event of interest, will be displayed in the views mentioned above.
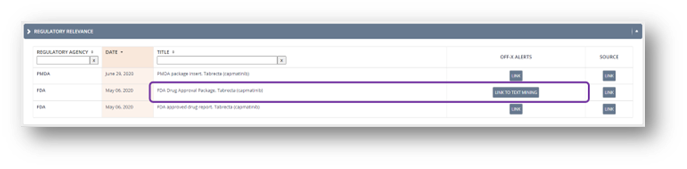
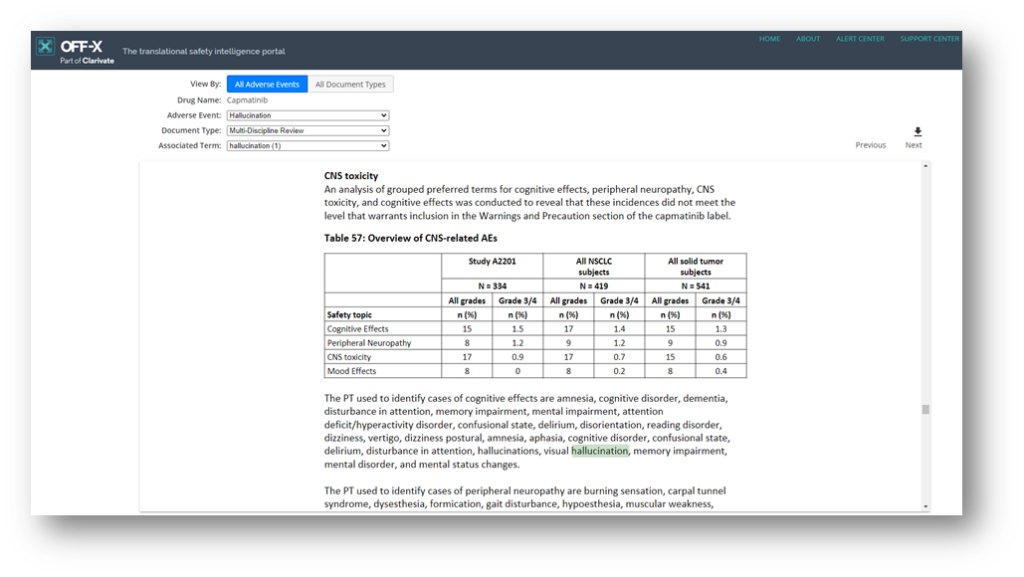
Given the great reception of our highly anticipated text mining approach to FDA Summary Basis of Approval documents (SBAs) released some months ago, a shortcut has been added next to each drug’s FDA approval package in the Regulatory Relevance section. Use this tool to easily navigate the adverse events found in each regulatory document via our text mining methods. You will find it in both drug and target’s Fast Facts.
The existing drug mapping between OFF-X and Cortellis Competitive Intelligence (CCI) has been updated and expanded. As a result, a higher number of OFF-X drug records are now linked to CCI. Simultaneously, a higher number of CCI drug records will include a snapshot of the safety profile of the drug based on the updated daily OFF-X content.