Comparative Drug Safety Evidence view enhancements
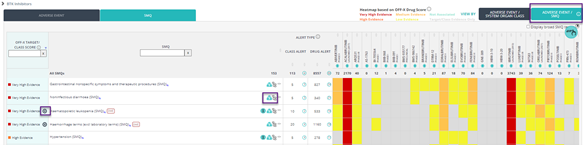
- New Adverse Event/SMQ view to navigate adverse events classified following Standardized MedDRA Queries (SMQ). SMQs include narrow and/or broad terms. Narrow terms are those that are highly likely to represent the condition of interest while broad terms include an expanded set of symptoms and findings that could be associated with the condition of interest. Narrow terms are displayed by default, but broad terms can be selected by applying the corresponding filter.
- Click on the corresponding icon next to each adverse event to explore the MedDRA hierarchy classification used in OFF-X (v24.1)
- Severity icon displayed next to those Adverse events to easily identify those adverse events classified/described in the original source as either serious or severe [Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or higher).
- On-target icon within OFF-X Target/Class score column when the adverse event is reported to be the results of the direct interaction between the drug and the target, when the drug is a selective monoclonal antibody, or when the toxicity has been observed in preclinical models of direct mechanistic evaluation, such as knockout studies in mice.
Safety Maps content update
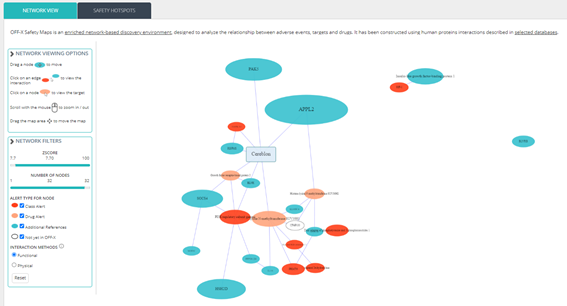
OFF-X Safety Maps is an enriched network-based discovery environment, designed to analyze the relationship between adverse events, targets and drugs and constructed using human proteins interactions. Use OFF-X Safety Maps to:
- Uncover potential toxicity and safety issues associated with new and emerging targets early in discovery by analyzing the neighborhood proteins & targets
- Prioritize target pipeline, considering potential serious safety events
- Analyze adverse event safety hotspots to better plan preclinical and clinical testing
Safety Maps content has been updated and now 12,156 target maps and 459 adverse events maps are available.
Real-World evidence dashboard: FAERS and JADER Q1 2021 data updated