Further integration of safety data extracted from FDA Summary Basis of Approvals (SBAs) via text mining methods in Drug record’s Master View, Translatability Dashboard and Real World Evidence (RWE) Analysis Dashboard to expand the coverage of regulatory safety intelligence by OFF-X.

Enhanced navigation of the FDA SBA text mining results: Identify all mentions of a certain adverse event of your interest in the different FDA SBA documents or go through all the adverse events identified in a specific document.

Enhanced filtering options in the RWE Analysis Dashboard: Use this new filter to prioritize new potential safety signals extracted from FAERS/JADER by excluding all adverse events included in drug labels or mentioned in the FDA SBAs.

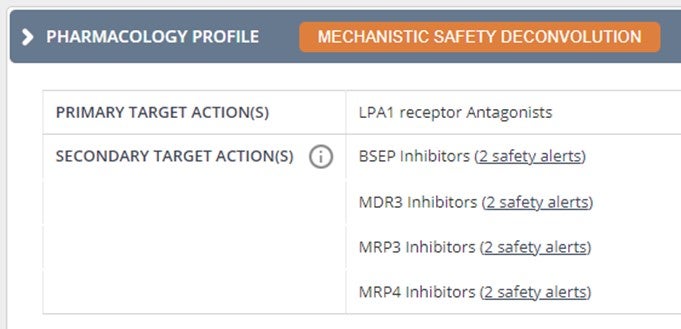
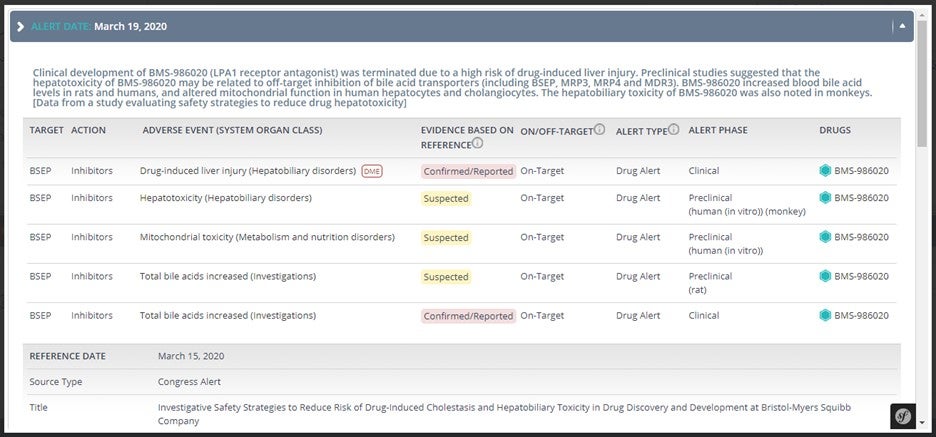
Safety alerts of Secondary Targets: Access easily the details and data sources reporting associations between off-targets of drugs and specific adverse events.
Navigate seamlessly between OFF-X and other Clarivate solutions, including Cortellis Drug Discovery Intelligence, Cortellis, Derwent Innovation and Web of Science, and vice versa.