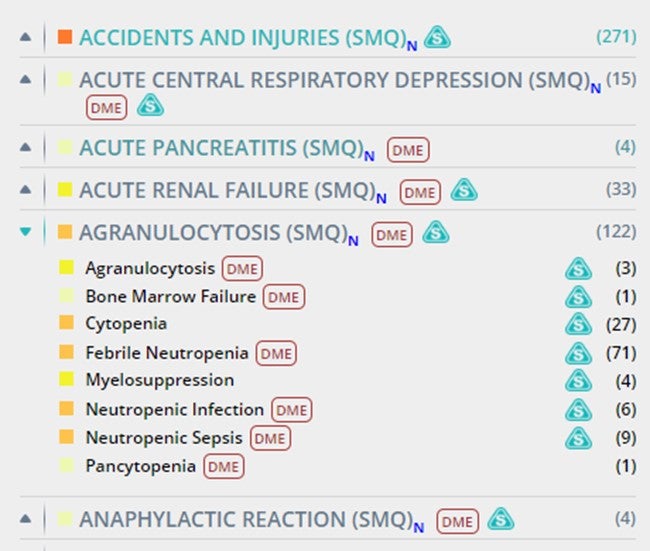
Easily navigate the adverse events of your drugs and targets of interest grouped by Standardized MedDRA Queries (SMQs) in the Safety Alerts section. A new view by SMQ, including both narrow and broad SMQs, has been added to the Safety Alerts view of drugs and target records to enhance the assessment of potential Pharmacovigilance signals.
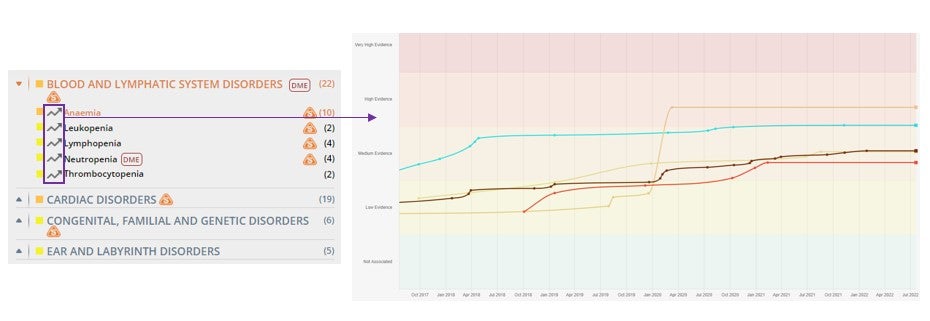
See the graphical evolution of the OFF-X Drug Score over time with a single click in the Safety Alerts view. You will be directed to the Mounting Evidence Chart view which will allow you to easily compare it with other drugs of the same or different classes.
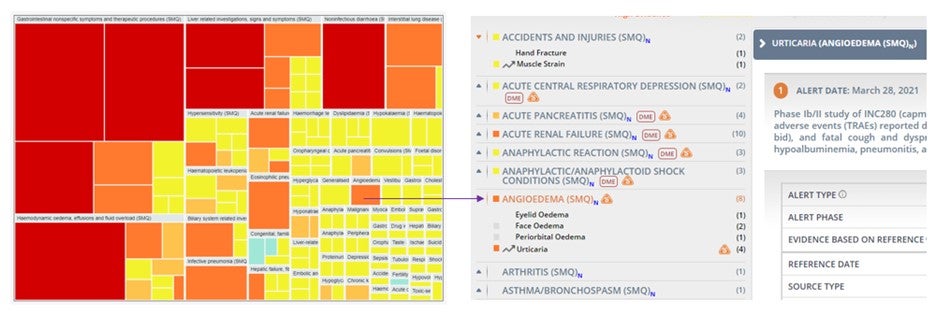
Get all the details behind the Heat Map view of drugs and targets. Click on your adverse event of interest in these high-level safety profile overviews to be automatically directed to the corresponding section in the Safety Alerts tab. Once there you will be able to explore all the reported insights, including links to the original data sources and analytics.
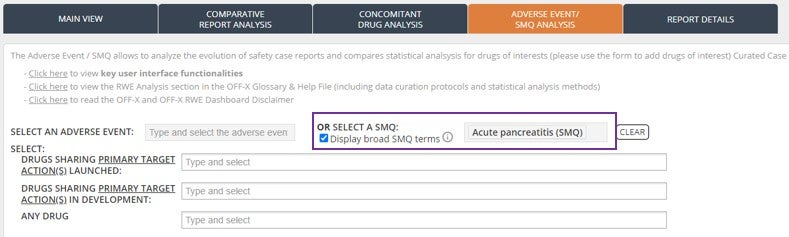
Expand the coverage of Standardized MedDRA Queries (SMQs) during your Pharmacovigilance signal assessments using the RWE analysis dashboard by automatically including broad SMQ adverse event terms.
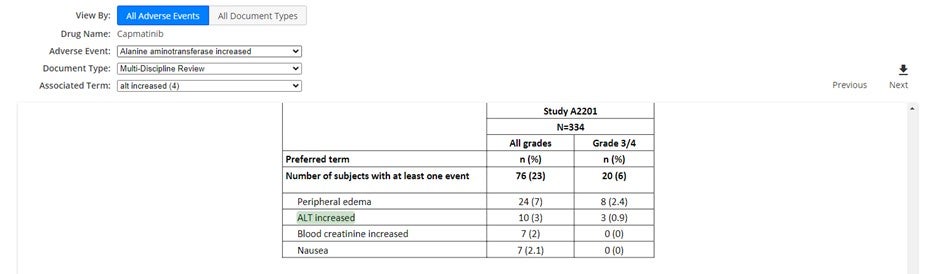
Enhanced coverage of FDA Summary Basis of Approval (SBA) documents via text mining: This feature is now available for over 1,000 drugs from the FDA archive, allowing you to easily identify all mentions of a certain adverse event in the different SBA documents or go through all the adverse events identified in a specific document.