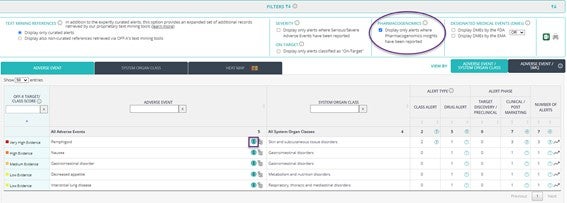
New Pharmacogenomics filter: Identify alerts with safety outcomes linked to the genomic profile of the patients according to the original source. Click on the icon next to each adverse event to explore the detailed information of the associated alerts.
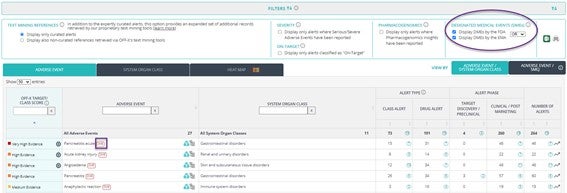
New Designated Medical Events filter: Designated Medical Events (DMEs) are adverse events that are inherently serious and often treatment-related. The new filter allows you to identify these adverse events of special interest for Regulatory Agencies in order to prioritize its monitoring. Click on the DME icon to explore the detailed information of the associated alerts.
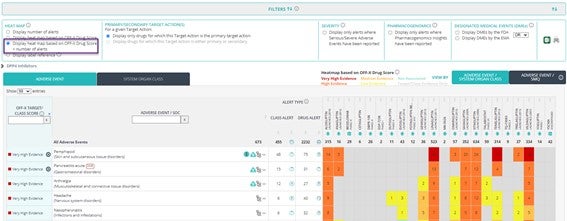
Comparative Drug Safety Evidence view enhancements
- New view of the heatmap based on OFF-X Drug Score including the number of associated safety alerts
Comparative Drug Safety Evidence view enhancements
- New view of the heatmap based on OFF-X Drug Score including the number of associated safety alerts
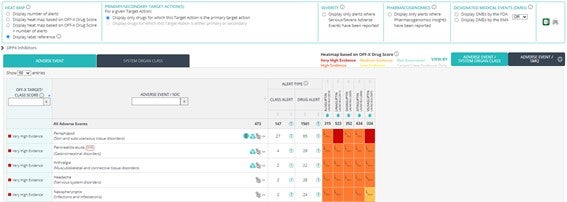
Drug and Target-action heatmap view enhancements:
- Number of associated alerts shown when hovering the mouse over a specific adverse events within the heatmap included in the Master view.

Real-World evidence dashboard: FAERS Q3 2021 and JADER Q2 2021 data updated