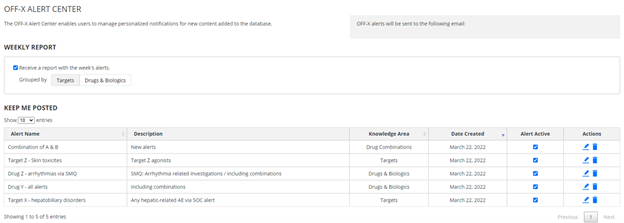
Custom Alert Center: Set up personalized keep me posted alerts for your drugs, drug combinations and targets of interest. You can select whether you want to be alerted of any new publication or only those associated with certain adverse events, SOCs or SMQs. These alerts can be easily set up from the corresponding record in OFF-X and managed from the new Alert Center.
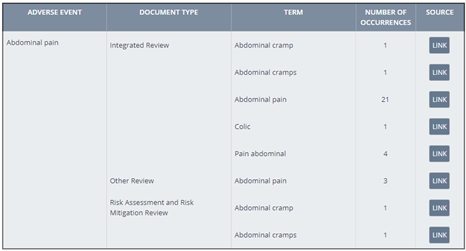
Mechanistic Safety Deconvolution: Save time when generating mechanistic hypothesis of the target potentially responsible for a given drug-adverse event association by assessing the safety profile of the drug’s primary and secondary target actions side by side. Find the Mechanistic Safety Deconvolution both in the drug records and the Analytics area.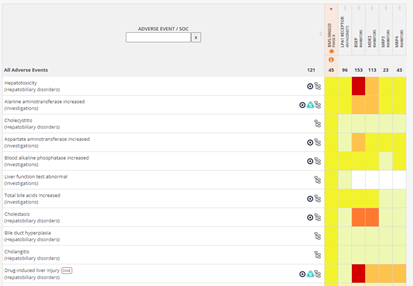
FDA Summary Basis of Approvals (SBAs): find safety data obtained from SBAs via text mining methods in the Drug record’s master view (view by adverse event) to complement the safety data manually curated from FDA labels.
Selectivity filter: Use this new filter in the drug comparative safety evidence table available both in drug and target records to easily identify drugs that are selective for the target analyzed.
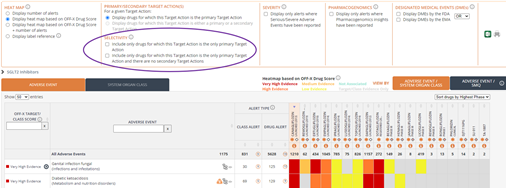
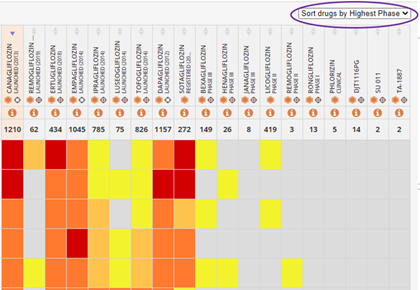
Sorting options: Gain additional competitive intelligence insights by sorting the drug comparative safety evidence tables by highest phase and selectivity.
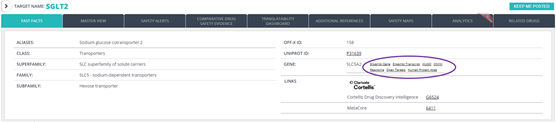
New external links: Explore additional information available in external resources thanks to the new links added to target records (Ensembl, HUGO, OMIM) and drug records (DrugCentral).