The clinical trials data available in OFF-X has been enhanced by including the clinical trial ID of each study and a link to clinicaltrials.gov to allow you to learn further details of the studies with a single click. Moreover, now you have the chance to easily identify safety alerts extracted from different publications covering the same clinical study. These are included in a new clinical trial record in OFF-X and allow you to identify the different data sources reporting results of each study that have been manually curated from clinical trial registries, publications in congresses, company communications or journal articles. As a result, you can monitor the timeline of the disclosure of results, from interim results reported in a congress or company website to the full details of the study published later. This view also includes pooled analysis of different studies or reviews of the safety profile of the drug or the drug class in which your study of interest has been included.
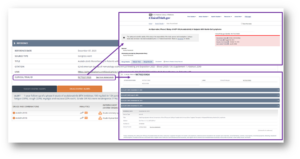
The clinical trials data described above has been leveraged to improve the OFF-X Drug Score. As a result, the latest version of the Score rule-based algorithm identifies curated safety alerts extracted from different publications reporting results from the same clinical study (e.g., results presented in two different congresses) and avoids computing them as additional evidence for each drug-adverse event association.
A series of recorded training sessions and webinars have been added to the Training Resources / Support Center section. Leverage these training materials to have an overview of OFF-X, get started with the tool or learn more about the latest key developments. In addition, two recorded webinars showcase different OFF-X use cases across all phases of drug R&D and postmarketing, one fully focused on pharmacovigilance and signal assessment/validation.