A new analytics page has been designed. A short description of each analytic tool has been provided allowing you to see all the available tools in a single screen. The different analytics have been classified based on the most typical use cases that OFF-X cover so you can select your use case of interest, shown at the top of the page sorted from discovery to pharmacovigilance, and identify the set of tools that better fit your needs.
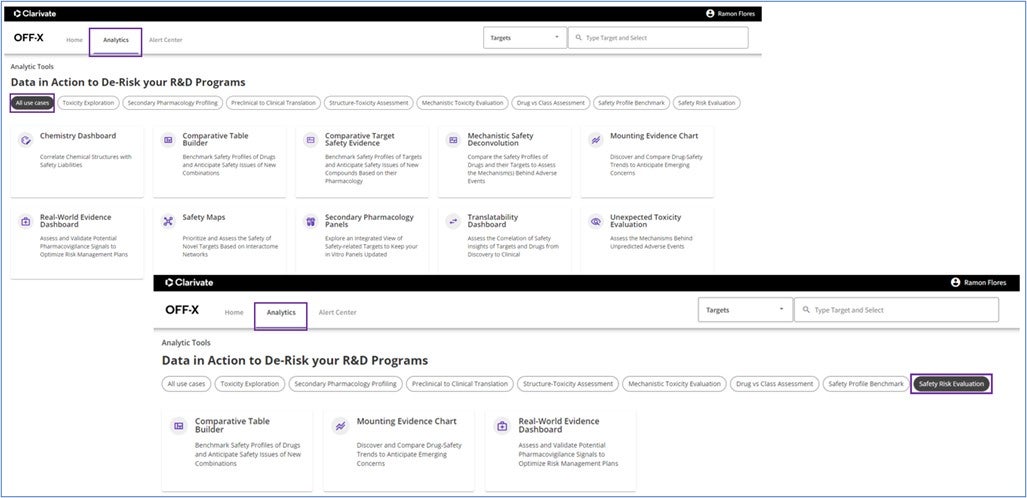
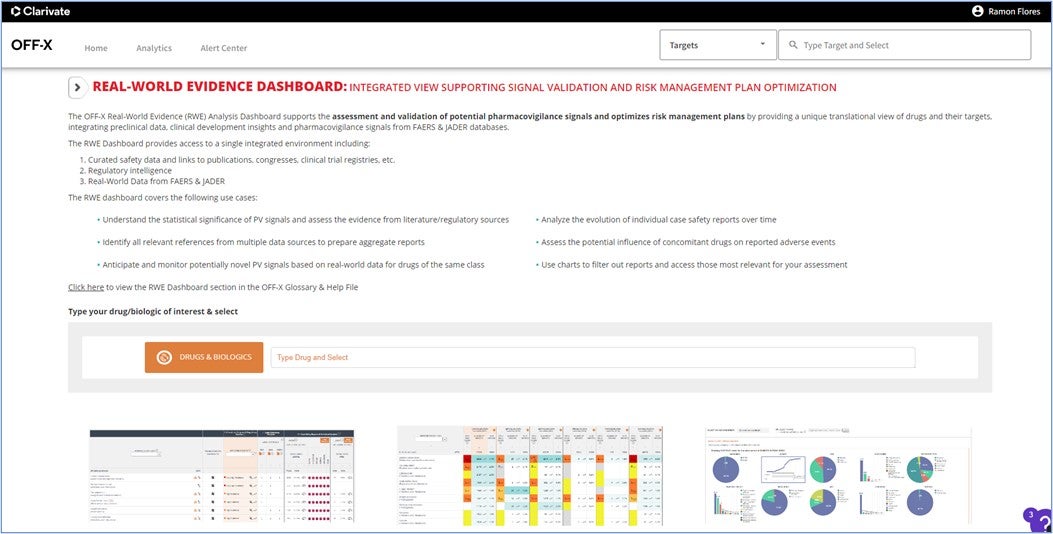
A new analytic tool that acts as a direct access to the Real World Evidence Analysis Dashboard has been created to enhance its usage in the context of pharmacovigilance use cases. This unique dashboard combines curated data from literature and regulatory agencies with FAERS and JADER data for the assessment and validation of pharmacovigilance signals.
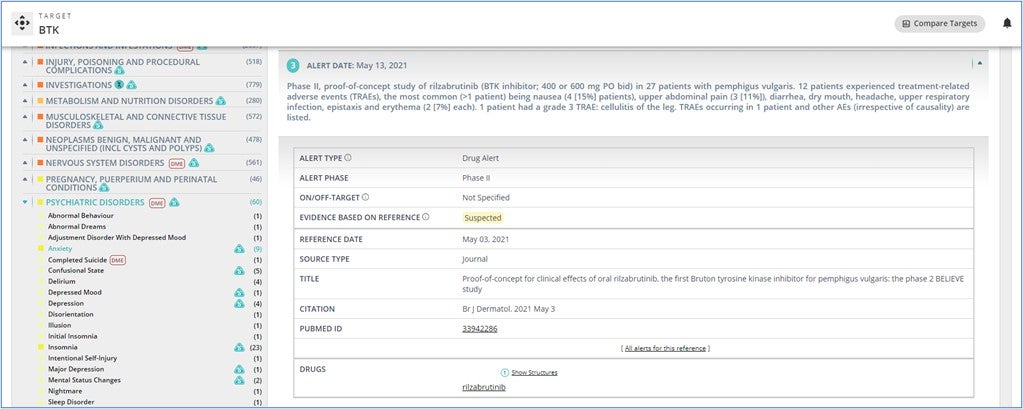
The header of the OFF-X page and the search bar have been re-designed. Moreover, a new sticky sub-header has been implemented indicating the name of the target, drug or adverse event record you are visiting. This functionality allows you to easily and quickly set up a keep me posted alert based on the content you may be reviewing anywhere in OFF-X using the corresponding bell icon. For targets, a quick access to the analytic tool that allows you to compare the safety profile of a given target with other targets, has been also included in the sub-header to give extra visibility to this key and unique functionality offered by OFF-X.

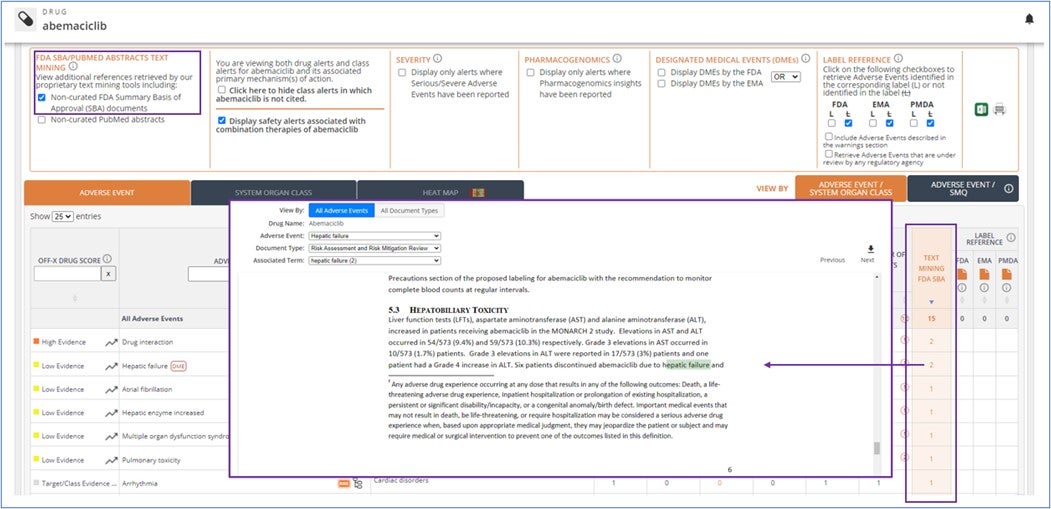
The Label Reference Analysis feature available for approved drugs has been enhanced. This tool allows you to easily identify the adverse events covered in approval documents (FDA, EMA, PMDA) for each drug and compare different drugs of the same or different classes. A new filter allows you to decide if you want to consider adverse events included in the warnings & precautions section of these documents but have not been reported with the drug. This improvement is based on customer feedback.
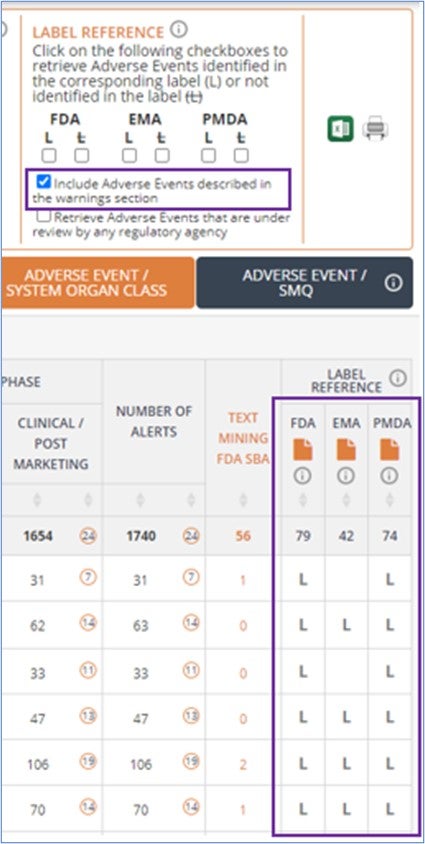
Moreover, the new version of the label reference analyzer now shows by default adverse events reported in the approval document when the drug was used in combination with other drugs. This is particularly useful for drugs typically used in combination such as antiretroviral therapies for which most of the safety data included in their approval documents is reported in combination. You have the chance to manage this option in the drug record’s master view using the previously existing filter below.

Safety insights obtained via text-mining from FDA Summary Basis of Approval documents are now shown by default in all views to give it more visibility. This data can be complementary to what has been manually extracted from FDA labels and was already available in OFF-X through a filter that allowed you to integrate it in the different tables and dashboards of the drug record.