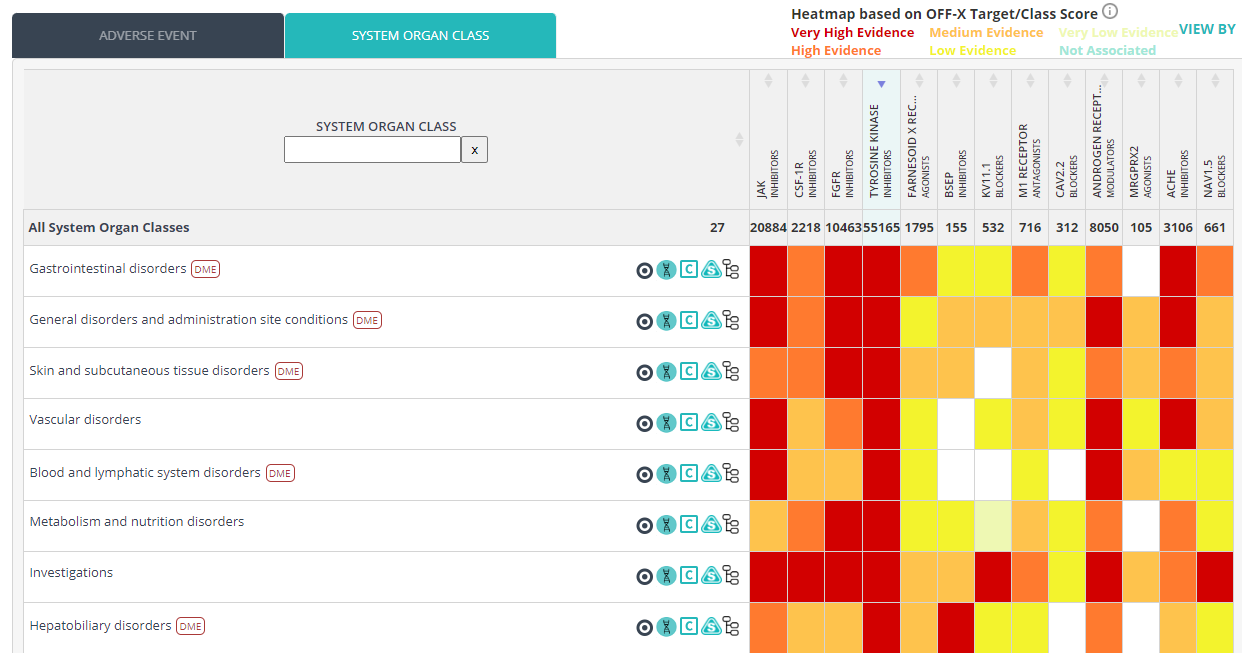
The rule-based algorithm behind the OFF-X Target/Class score, a measure of the evidence linking targets and adverse events, has been enhanced to leverage the latest metadata incorporated into OFF-X:
- Causality. Whenever a publication reports a treatment-related adverse event it will be given a higher weight than an adverse event with no reported causal relationship with the treatment.
- Higher weight has been given to drug-adverse event pairs directly associated with clinical holds, clinical trial terminations or drug development discontinuations.
Moreover, we have also made some modifications based on the feedback we have received from OFF-X users:
- Slight reduction of the weights given to safety alerts based on regulatory communications when these are not drug approval packages.
- Slight reduction of the weight given to class effects included in the Warnings section of approval packages as these may be included in the approval packages of different drugs of the same class.
- Slight increase of the weights given to preclinical findings to give more prevalence to toxicities that might have been associated with the discontinuation of the development before entering clinical development.
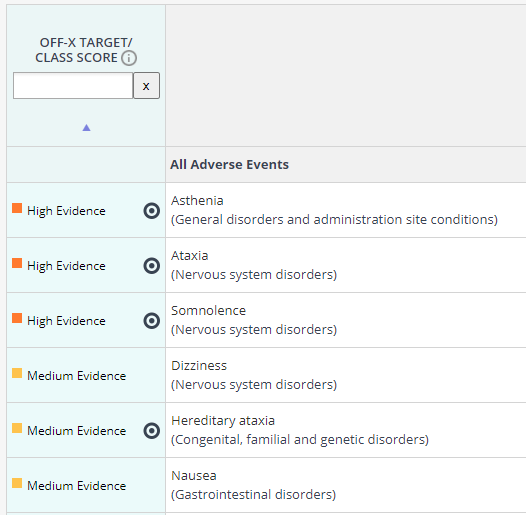
Some of the most popular use cases leveraging the OFF-X Target/Class Score include:
- Generation of mechanistic hypothesis of toxicities by assessing the safety profile of a drug and its primary and secondary target actions side by side.
- Comparison of safety profiles of different targets for the same indication, identify potential advantages in terms of safety for repurposed targets, etc…
- Identification of toxicities that could be exacerbated by targeting different pathways via new drug combinations
- Anticipation of potential safety issues of novel compounds (even before being tested in animals) based on their experimental secondary pharmacology profile
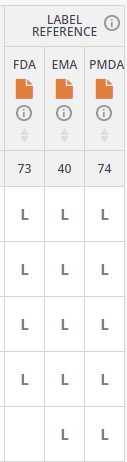
Access safety data for all the 1900+ drugs marketed in the US, manually extracted from the archive of FDA approval packages. OFF-X’s unique approach allows you to save time when comparing the safety insights from FDA, EMA and PMDA approval documents via the Label Reference functionality available in drug record’s master view, Real-World Evidence analysis dashboard and the different comparative drug safety tables. This is part of a two-year project that will lead us to complete the analysis of archives of approval packages by the PMDA (finished in 2022), FDA, EMA (scheduled for the end of Q4) and UK’s MHRA (Q1 2024).