The OFF-X coverage of discontinued drugs has been enhanced with an extensive analysis of journal articles from the last 25 years. As a result, the adverse events reported during preclinical and clinical development of such molecules have been captured, even when safety was not disclosed to be the reason behind the discontinuation of these drugs. This safety intelligence is of utmost importance to complement that obtained from drugs in the market or under active development to provide a full picture of the safety profile of target classes and help anticipate potential risks for novel drugs.
This is part of a continuous effort to keep adding relevant legacy data to OFF-X. In parallel with the curation of this content, we are working on an enhanced version of the OFF-X Drug Score that will leverage drug-adverse events pairs effectively linked with clinical discontinuations, clinical holds, or withdrawals from the market to provide higher evidence to such associations (scheduled for June 14th).
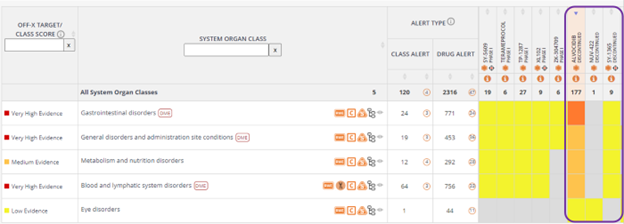
Two new reviews have been recently added to the Secondary Pharmacology Panels analytic tool. Now, you can find up to 8 relevant publications reviewing secondary pharmacology targets and their associated safety liabilities. Leverage the different views provided by this tool to identify the most commonly discussed off-targets or the latest recommended targets that should be tested in vitro before submitting a New Drug Application (NDA) to regulatory bodies. You can also assess the most relevant targets to build in-vitro panels to anticipate specific organ toxicities based on the secondary pharmacology of new lead candidates.
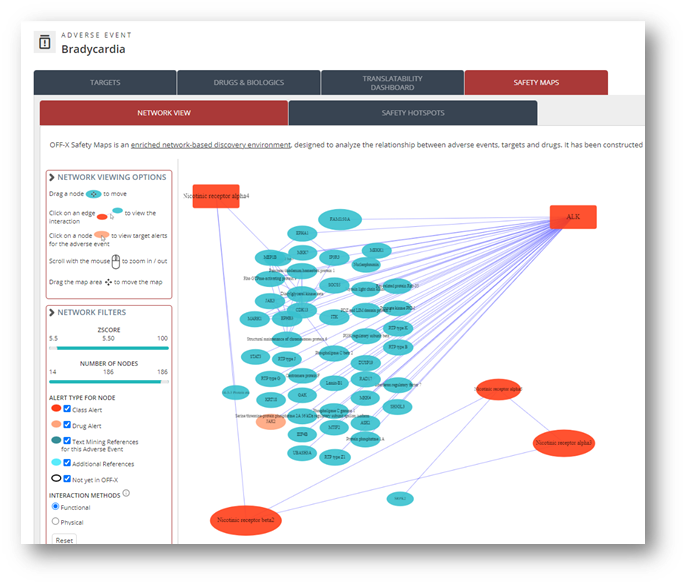
All OFF-X safety maps have been rebuilt based on the latest updated version of the different human interactome databases used to construct them. You can now access more than 12,500 target-centric and close to 600 adverse event-centric maps. This interactome approach is particularly interesting for novel targets as it allows you to anticipate potential toxicities based on the known safety profile of their surrounding proteins.
200 additional FDA labels approved between 1980-1999 have been added to OFF-X. New safety data manually extracted from FDA labels of drugs approved before 1980 will be incorporated into the portal every week during the next 3 months. This is part of a two-year project that will lead us to complete the analysis of archives of approval packages by the PMDA (finished in 2022), FDA (scheduled to be completed in Q3), EMA (Q4) and UK’s MHRA (Q1 2024).
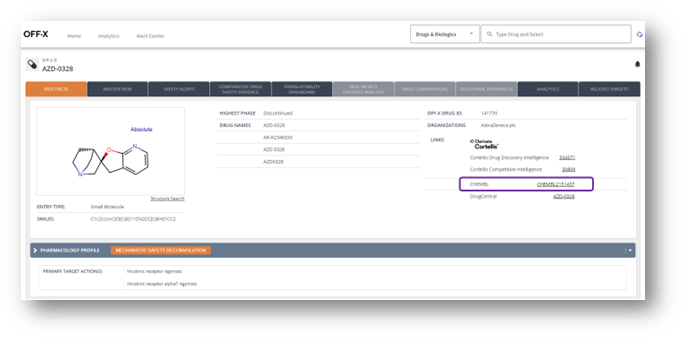
The number of external links to ChEMBL has been increased. We have updated our mapping to allow the creation of more than 400 new links from OFF-X drug records to the corresponding drug records in ChEMBL.