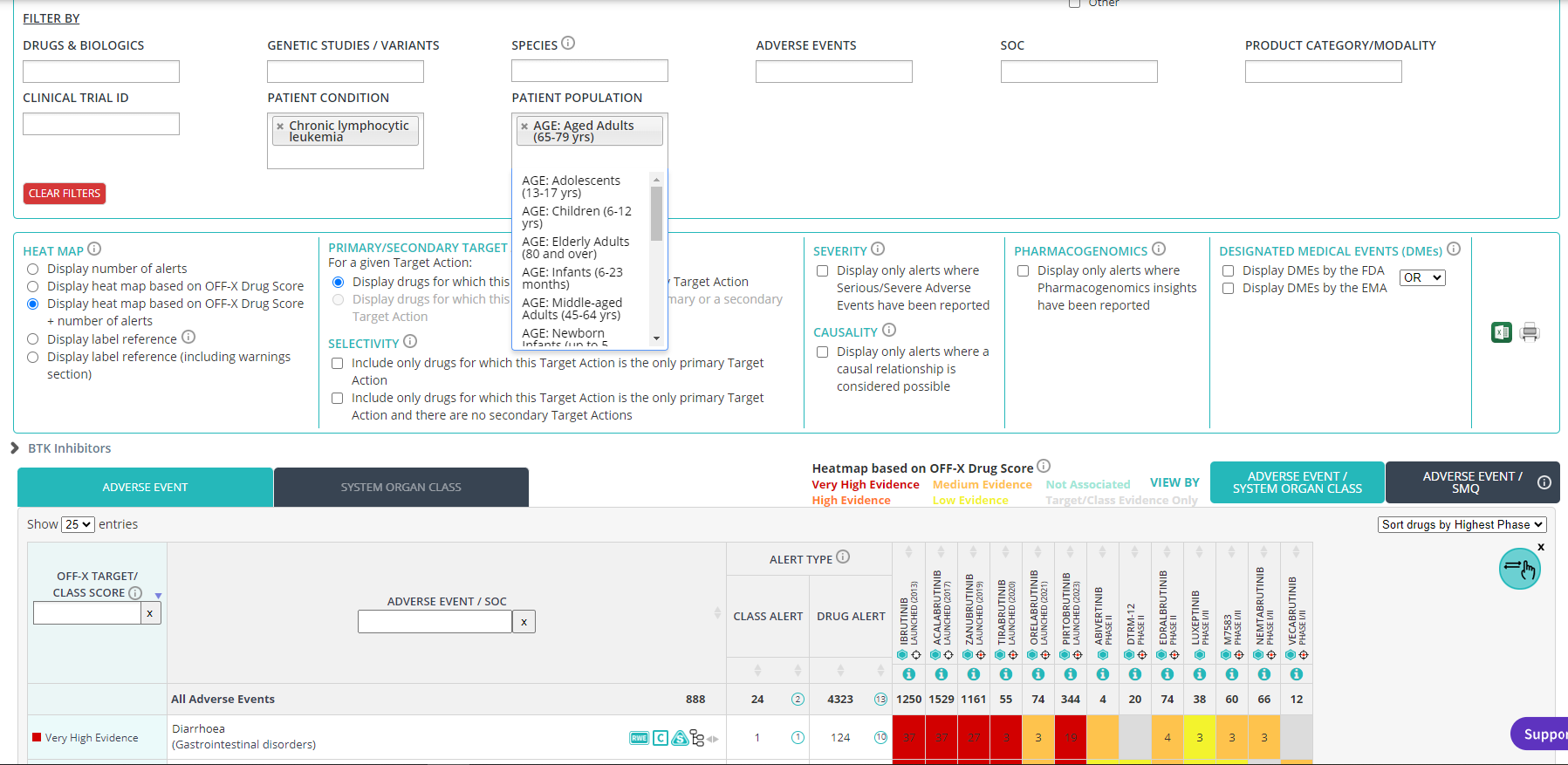
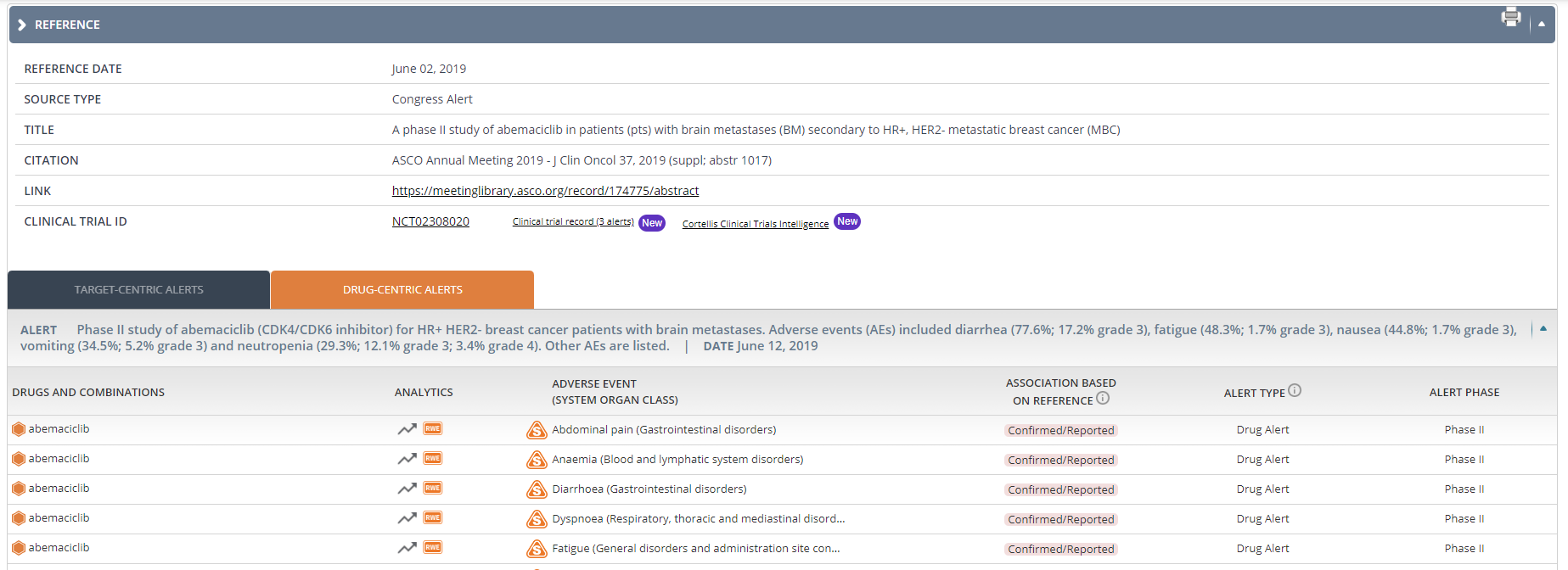
Access new clinical trial metadata to enable the safety assessment of treatments for diverse populations. The characteristics of the patients enrolled in a clinical trial may determine at some extent the safety outcomes of the study. You can now filter by a specific clinical trial ID, patient population (age, ethnicity, gender, volunteers) and/or condition in any OFF-X view to identify those adverse events reported with a given drug when administered to certain patients or to compare the safety profile of different drugs, or targets, when used with the same type of patients.
New data associated with clinical studies is now available in each Clinical Trial record page in OFF-X, including patient condition, patient population and detailed information about the treatment such as the doses and administration routes for each drug.
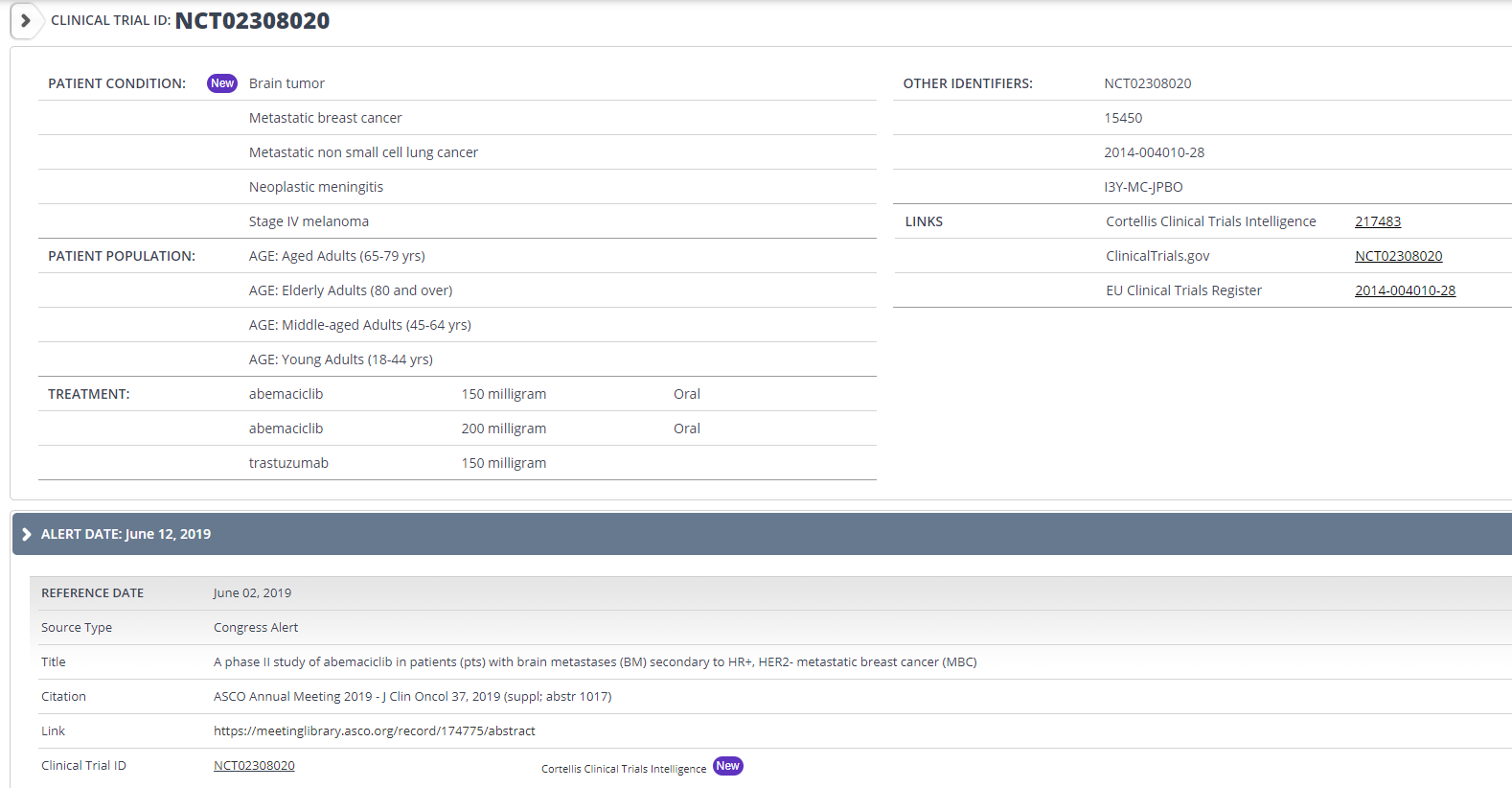
Cross-linking with Cortellis Clinical Trials Intelligence (CTI) to allow seamless navigation from OFF-X to CTI to easily gain additional insights about clinical studies, including inclusion criteria, efficacy endpoints, etc… Likewise, CTI users will find links to OFF-X to assess the safety intelligence extracted from each clinical study.
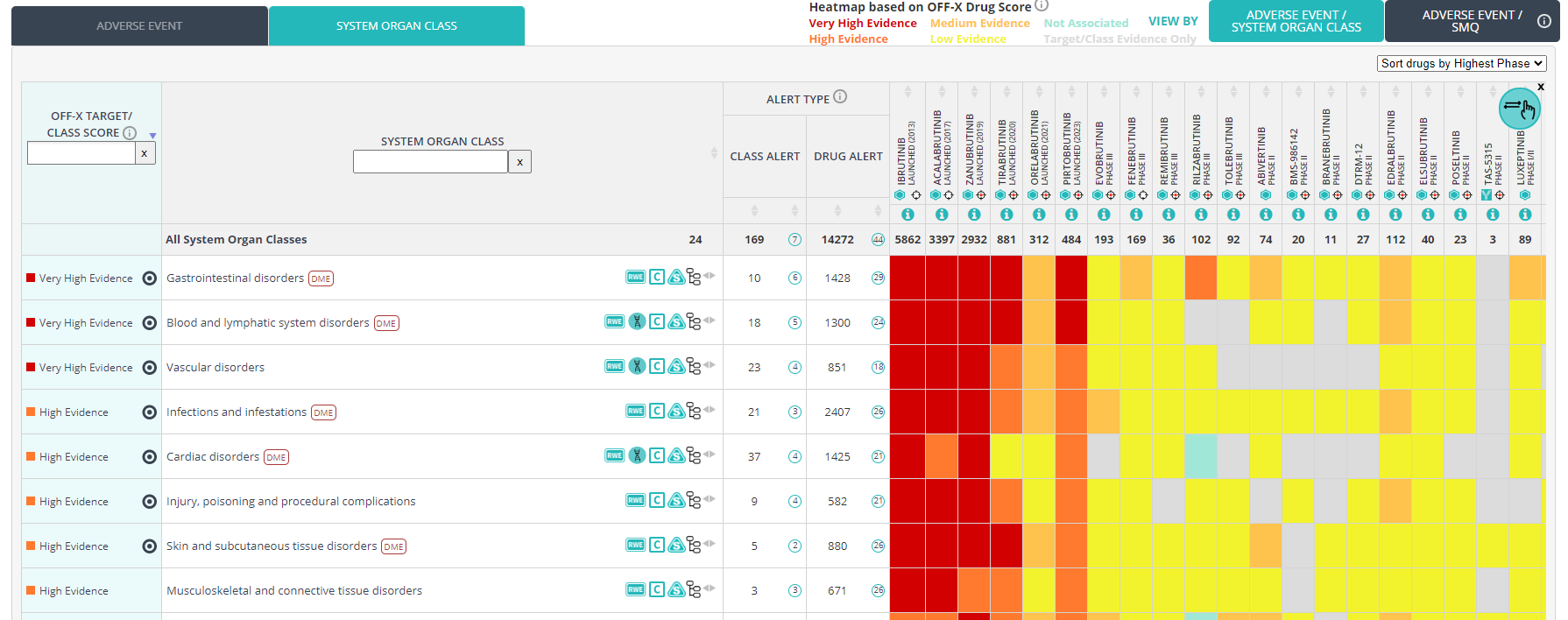
The rule-based algorithm behind the OFF-X Drug score, a measure of the evidence linking drugs and adverse events, has been enhanced to leverage metadata recently incorporated into OFF-X:
- Causality. Whenever a publication reports a treatment-related adverse event it will be given a higher weight than an adverse event with no reported causal relationship with the drug.
- Higher weight has been given to drug-adverse event pairs directly associated with clinical holds, clinical trial terminations or drug development discontinuations.
Moreover, we have also made some modifications based on the feedback we have received from OFF-X users:
- Slight reduction of the weights given to safety alerts based on regulatory communications when these are not drug approval packages.
- Slight reduction of the weight given to adverse events included in the Warnings section of approval packages when these have not been reported yet with the corresponding drug. Given that some drugs might get warnings due to serious safety liabilities of other members of the class, this modification will allow a better assessment of the safety profile of newer generation drugs.
- Slight increase of the weights given to preclinical findings to give more prevalence to toxicities that might have been associated with the discontinuation of the development before entering clinical development.
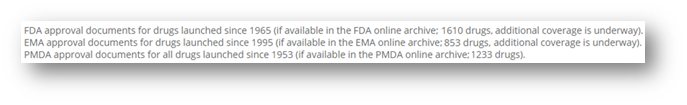
All the safety intelligence extracted from FDA labels approved since 1965 is now available in OFF-X. This is part of a two-year project that will lead us to complete the analysis of archives of approval packages by the PMDA (finished in 2022), FDA (scheduled to be completed during Q3), EMA (Q4) and UK’s MHRA (Q1 2024).