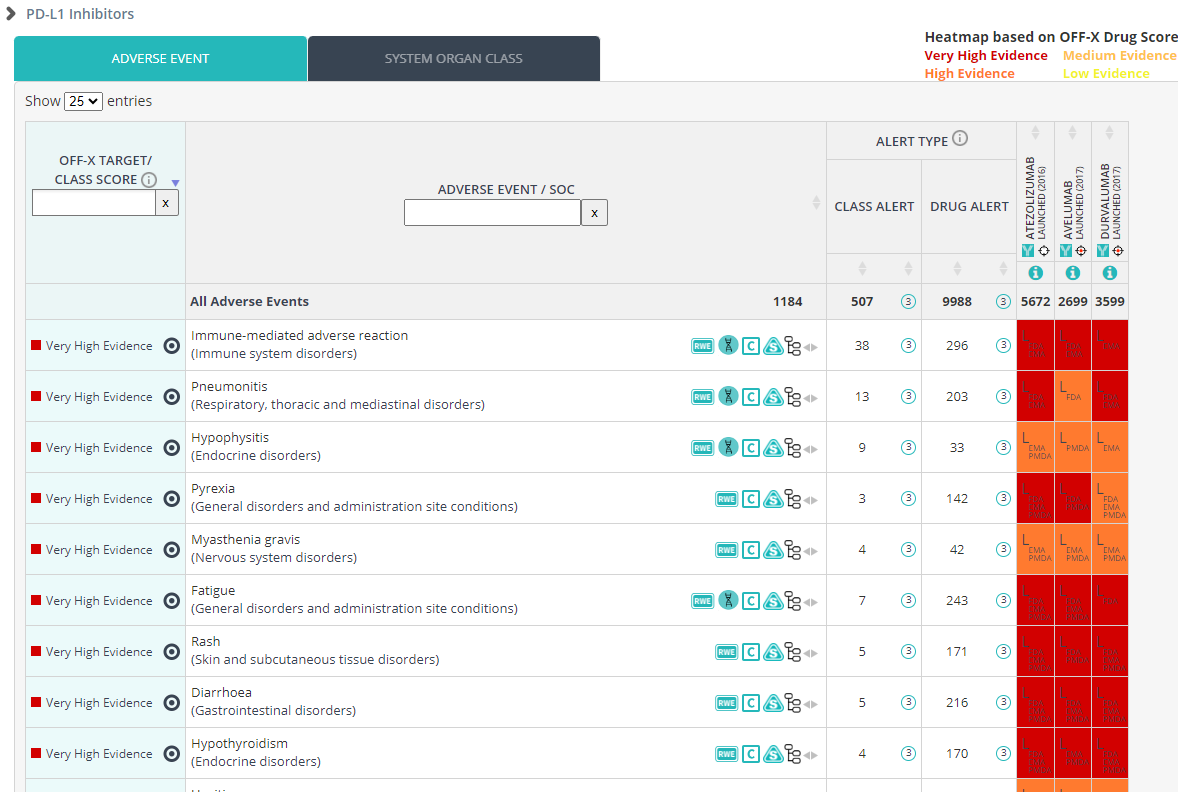
Assess the safety profile of approved and investigational gene therapies based on the type of virus used to deliver the genetic material. Find now in OFF-X the safety data of all gene therapies classified according to their viral victor. Search for your viral vector of interest in the Target area to explore the safety insights of the corresponding class, including the OFF-X Target/Class Score that will compute in real time the degree of evidence associated with each class-adverse event pair. Use the latter to benchmark the safety profiles of the different viral-vector classes. This approach is aligned with the way safety data for other modalities, such as cell therapies or vaccines, are grouped together in OFF-X, facilitating the assessment of potential class effects as well as differences between members of the same class.
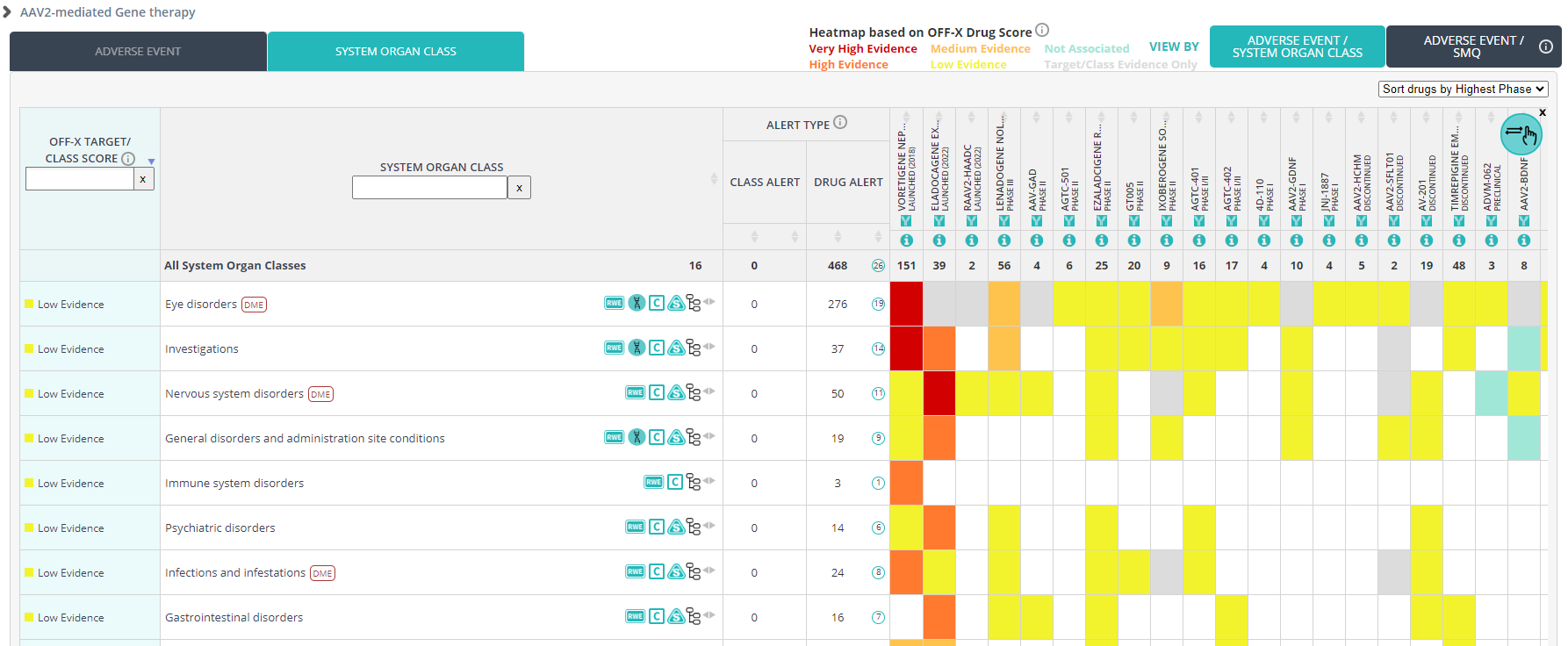
The Additional References section of both Target and Drug records has been updated. New, improved text mining/machine learning methods have been implemented to identify potentially relevant articles indexed by PubMed along with the key terms found in the title/abstract, article’s keywords, and MeSH terms. This is the same methodology used in our core OFF-X editorial workflows to prioritize the content that is manually curated and added to OFF-X daily.
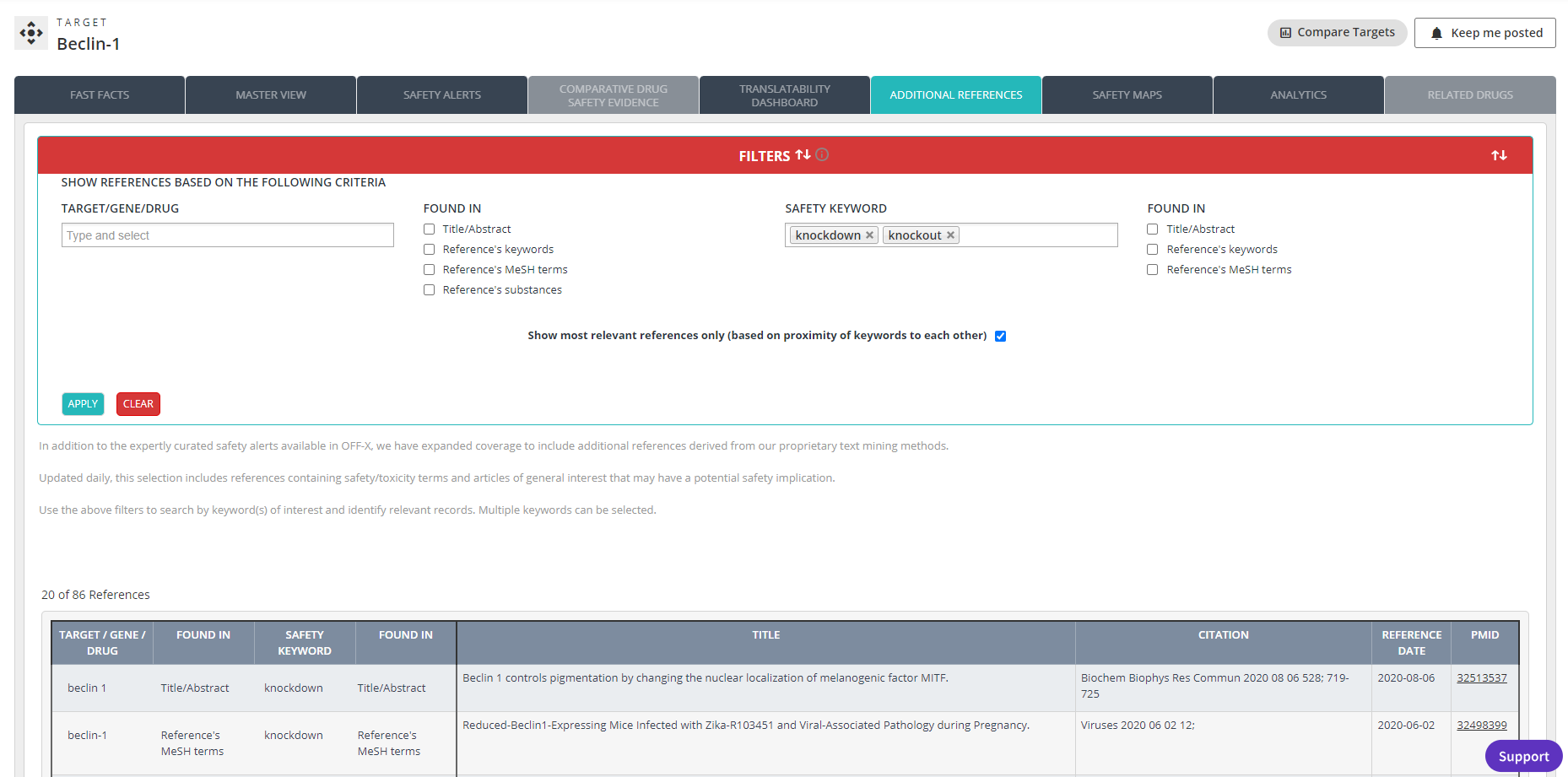
The most relevant results found via this approach are also incorporated in other OFF-X views such as the master view, the translatability dashboard, or the Real-World Evidence analysis dashboard. Leverage it to find relevant PubMed publications associated with your drugs and targets of interest to complement the safety intelligence extracted from manually curated safety alerts. The combination of both approaches provides a comprehensive coverage of what has been indexed in PubMed for drugs and targets.
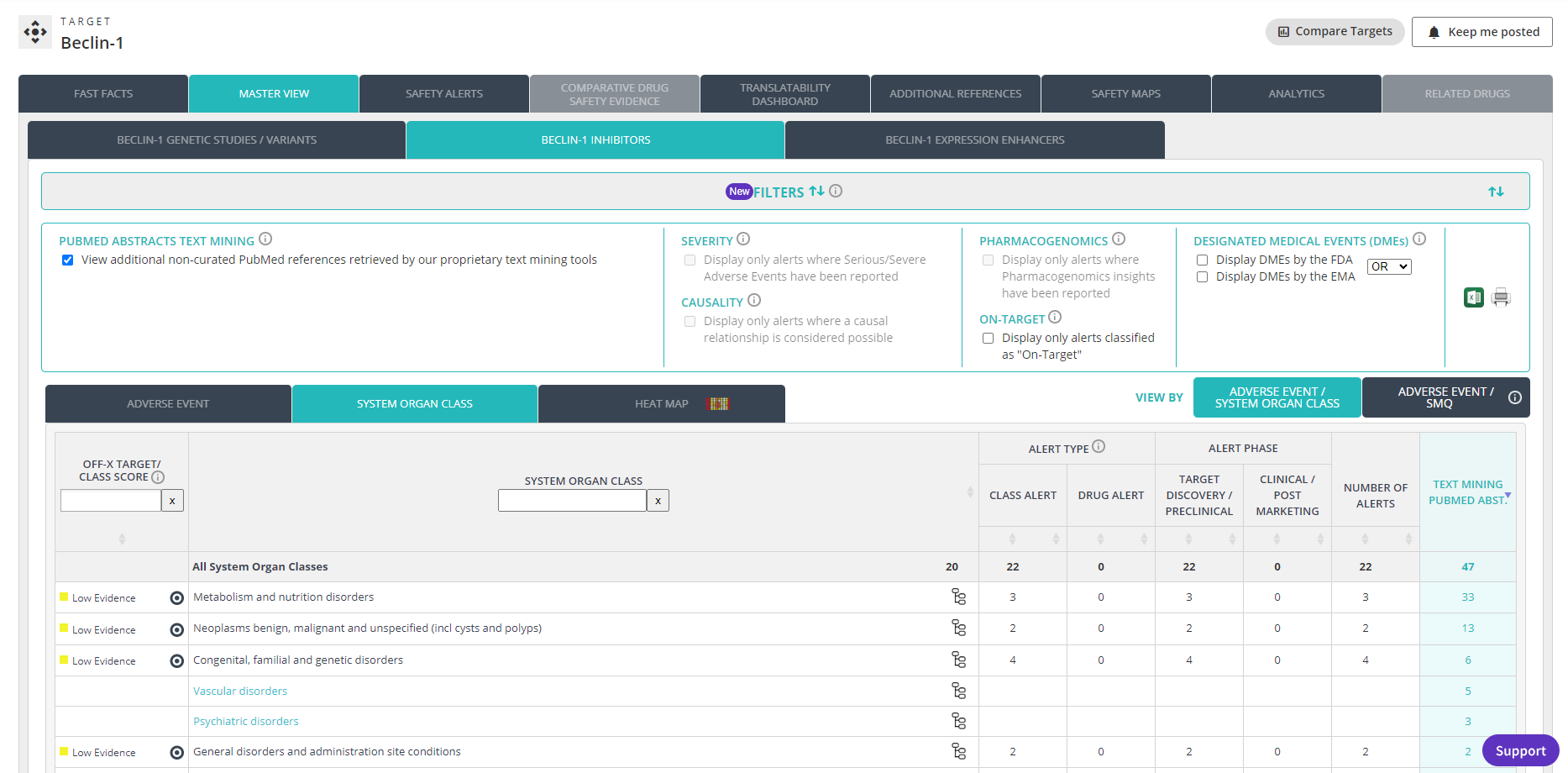
Access safety data for the 1200+ drugs marketed in Japan, manually extracted from the archive of PMDA approval packages. OFF-X’s unique approach allows you to save time when comparing the safety insights from FDA, EMA and PMDA approval documents via the Label Reference functionality available in drug record’s master view, Real-World Evidence analysis dashboard and the different comparative drug safety tables. As part of our continuous enhancement of the breadth and depth of OFF-X curated data, during 2023 we will be expanding our legacy coverage of FDA labels and EMA EPARs.