We are thrilled to announce the release of a series of enhancements to the beta version of the new OFF-X platform:
Enhanced export functionalities: All the existing excel exports in the legacy OFF-X are now available in the new platform. The new look and feel of the resulting tables has been designed to maximize the value that can be extracted from OFF-X via these specific pieces of safety intelligence. Several new additions of data, including a date and time stamp, have been included in such exports following user feedback.
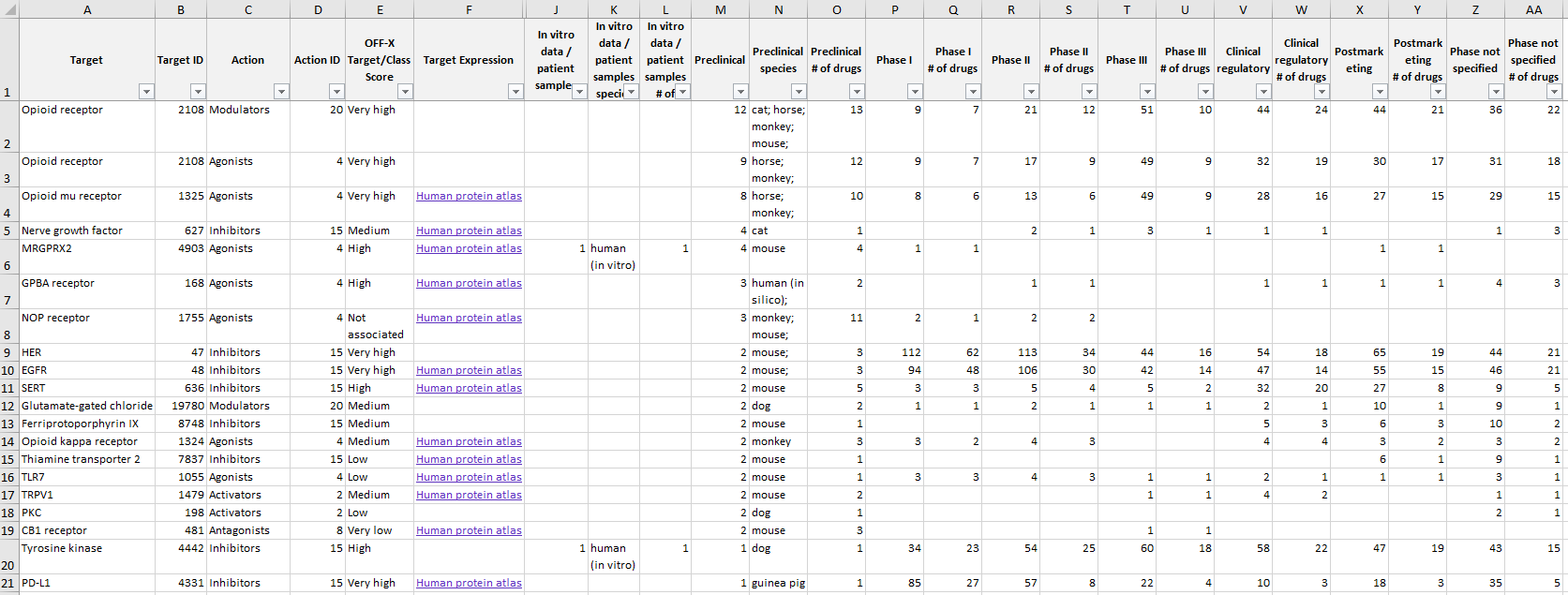
Moreover, the translational safety view (target, drug and adverse event areas) is now available as an xlsx file for the first time.
Additional export options will be available in forthcoming releases, including safety alerts and Real-World Evidence analysis in the new platform. These sections can not be exported using the legacy OFF-X platform.
Email notifications: You can now set up personalized keep me posted notifications for your targets and drugs of interest using the new platform. You can select whether you want to be alerted of any new publication, regulatory label changes, new adverse events or certain adverse events, SOCs or SMQs.
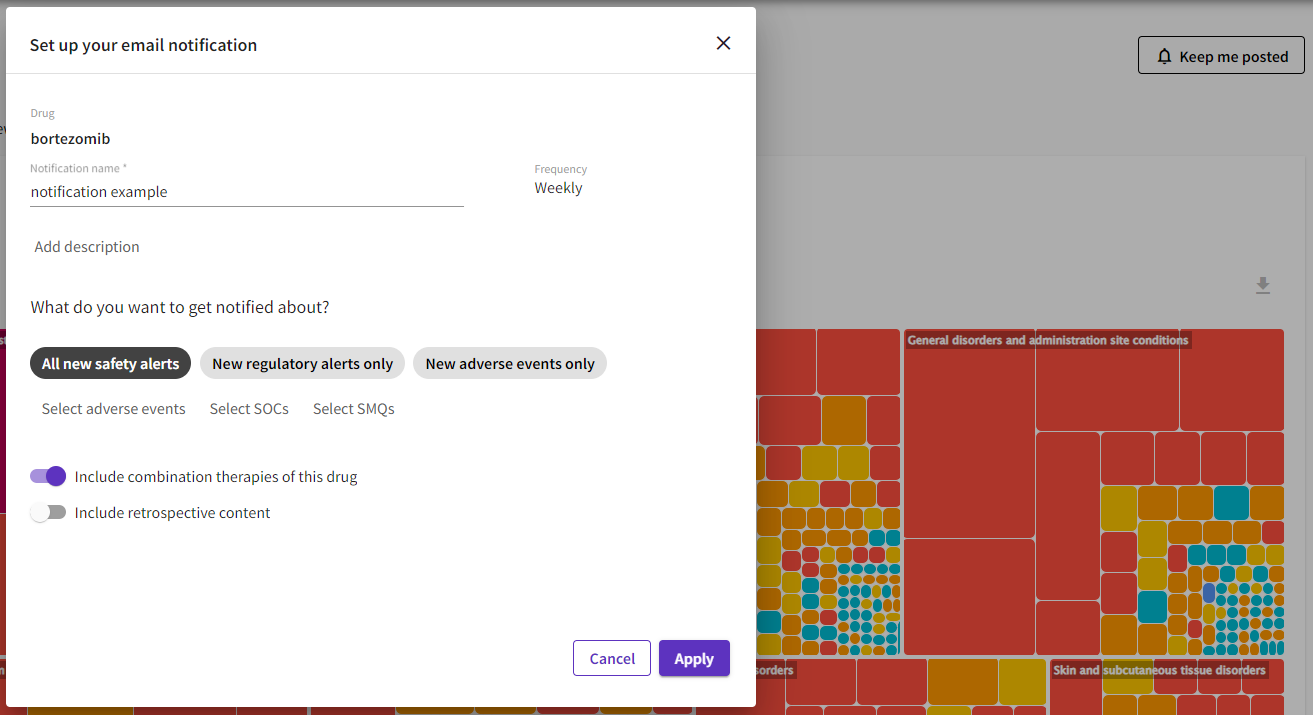
These notifications can be easily set up from the corresponding drug or target record and edited and managed using the Notification center available at the top of all OFF-X pages. Please note that all your previous notifications will be shown in the Notification center of the new OFF-X platform.
With the implementation of the export and alert notifications, the beta version of the new OFF-X platform allows you an end-to-end assessment of targets, drugs and adverse events by complementing the previously existing functionality with the option to export the outcome of your analysis and setting up alert notifications to be promptly informed each time relevant information is available in OFF-X.
Contextual FAQs: Concise information about each page and view of the new OFF-X platform can now be easily displayed by clicking on the FAQs button available at the bottom of the pages.
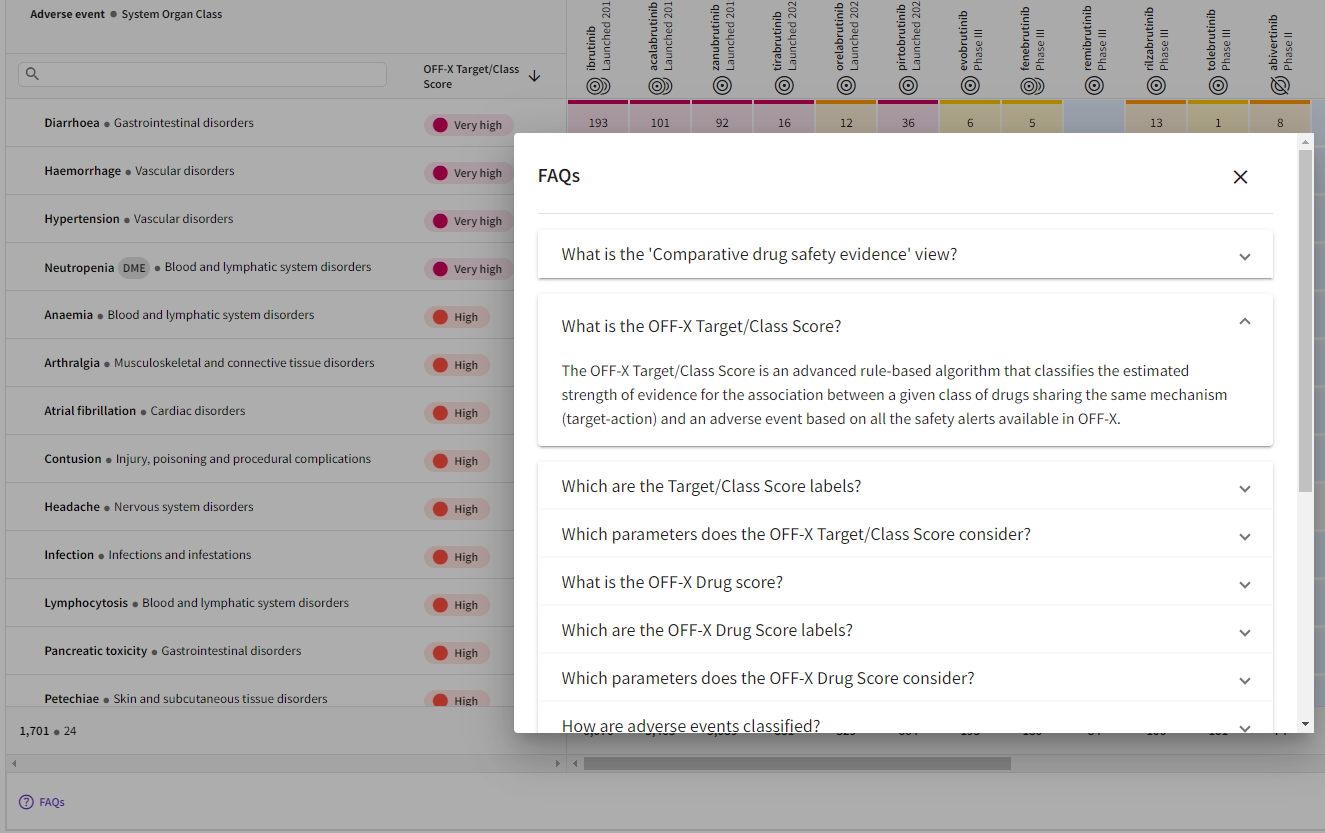
Extended contextual help, shown as short and cross-linked articles, will be available in future releases with the deployment of the new OFF-X Knowledge Base.
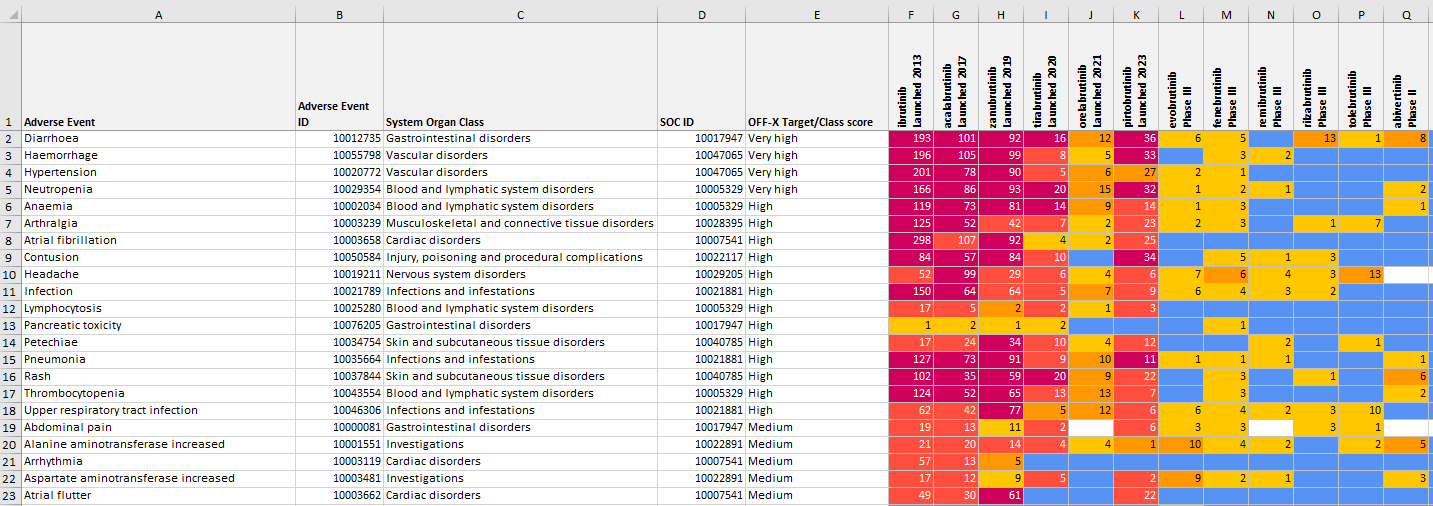
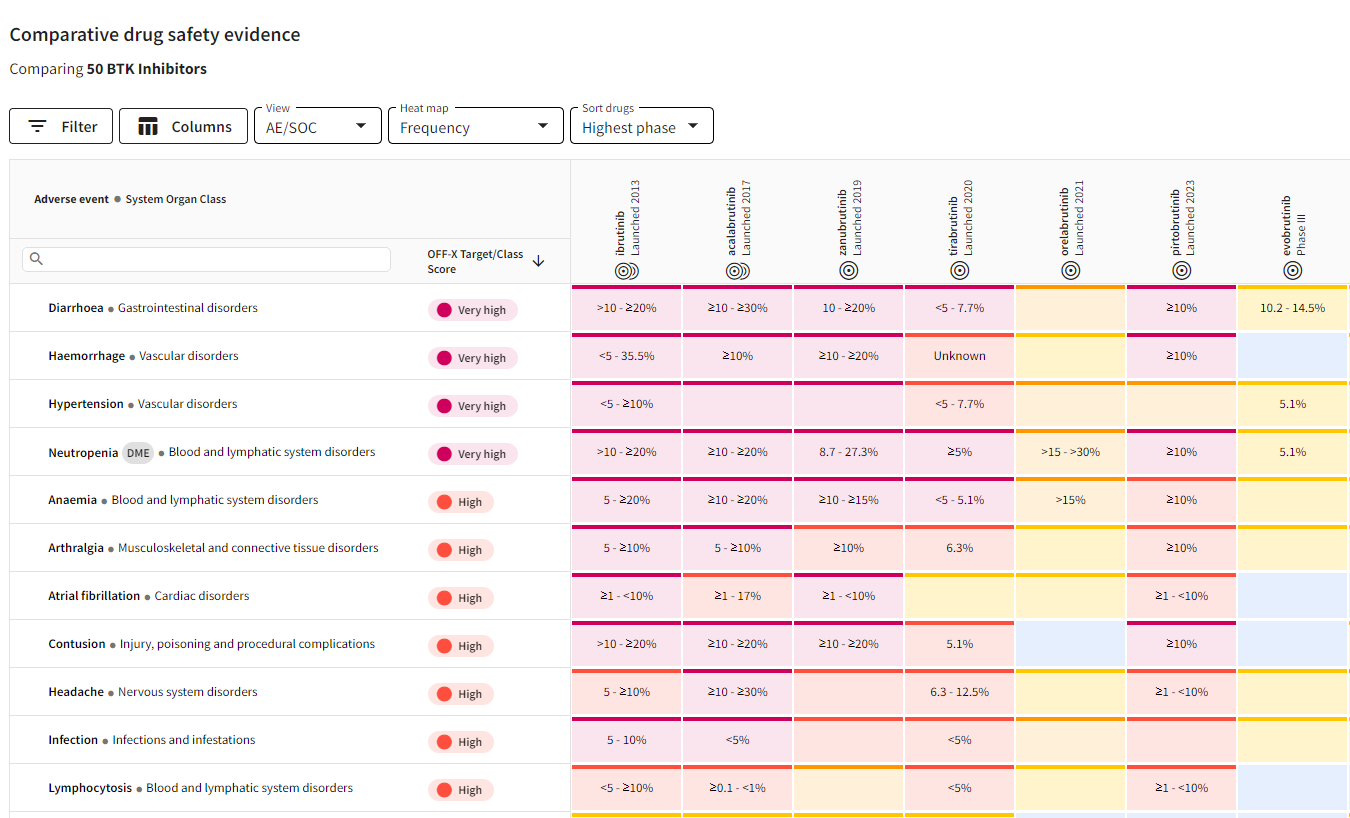
Comparative drug safety evidence views with adverse event frequencies: A new heat map view has been developed in the comparative drug safety evidence views allowing you to compare the ranges of frequencies reported for each drug and adverse event in clinical trials. This approach allows a new, quantitative benchmarking of the safety profile of drugs.
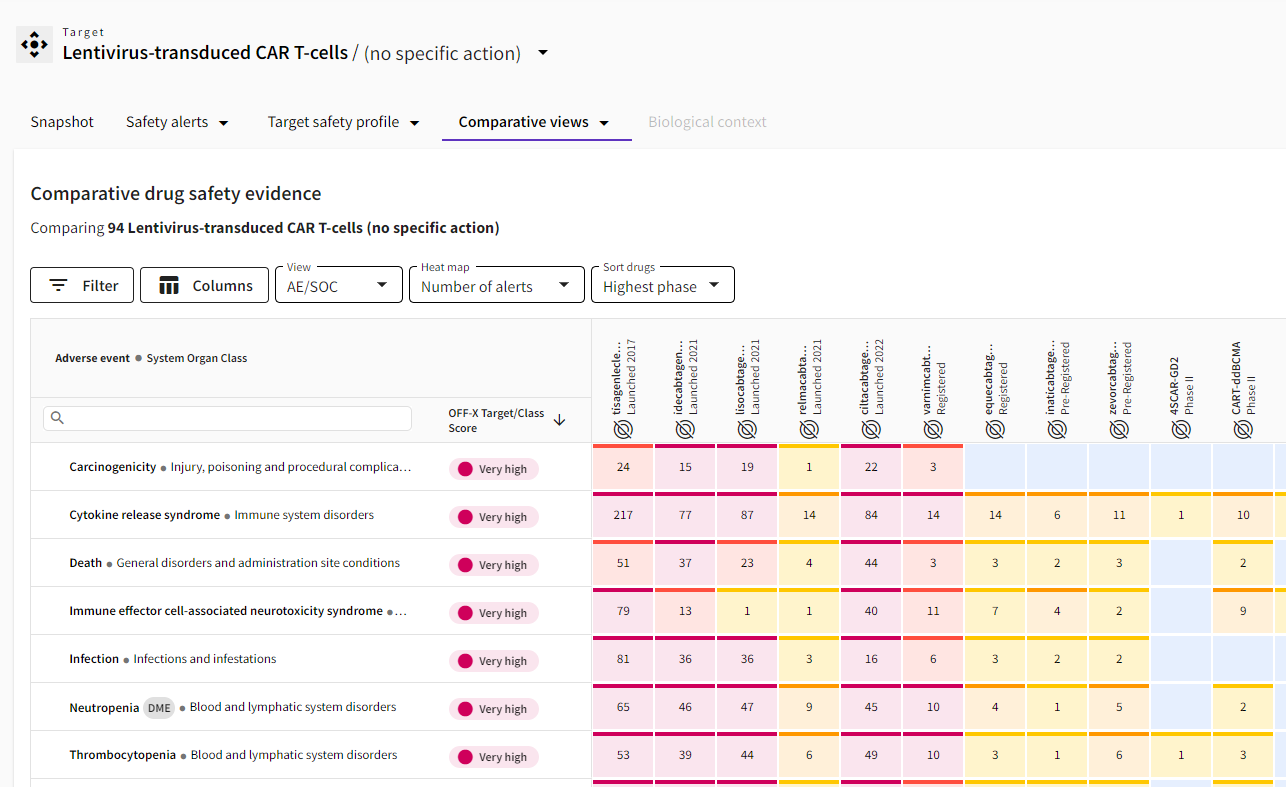
New grouping of CAR-T cell therapies: Assess the safety profile of approved and investigational CAR-T cell therapies based on the type of virus used to transfer the genetic material. Search for your viral vector of interest in the Target area to explore the safety insights of the corresponding class of cell therapies, including the OFF-X Target/Class Score that computes in real time the degree of evidence associated with each class-adverse event pair. Use the latter to benchmark the safety profiles of of CAR-T cell therapies based on different viral vectors. This approach is aligned with the way safety data for other modalities, such as gene therapies or vaccines, are grouped together in OFF-X, facilitating the assessment of potential class effects and understanding the origin of toxicities.
More coming soon! We will continue to add new features to the new OFF-X platform in the coming months. Both platforms will coexist until June 2024, when the legacy OFF-X platform will be decommissioned.