Use the new OFF-X causality metadata & filter to retrieve easily adverse events reported as treatment-related in the original data sources. Save time identifying these publications during the assessment of safety signals, the preparation of aggregated reports or to anticipate potential liabilities of new drugs based on other members of the class.
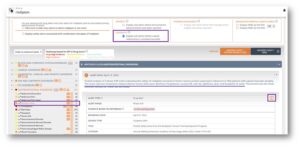
Adverse event frequencies extracted from clinical trial registries and approval documents by the FDA, EMA and PMDA are now available in OFF-X. These values complement the OFF-X Drug Score, a qualitative measure of the evidence associated with each drug-adverse event association. Leverage the synergy of both approaches to anticipate safety issues during the design of new clinical trials, the assessment of novel drug combinations or to benchmark competitor’s drug safety profiles.
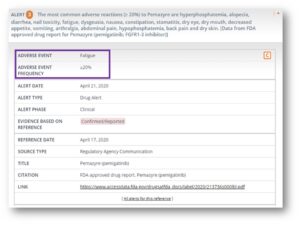
A new column summarizing the range of adverse event frequencies reported in the different clinical studies analyzed has been included in each drug record’s master view. Click on these value ranges to quickly retrieve all the details of each study, sorted from lowest to highest drug-adverse event frequency reported.
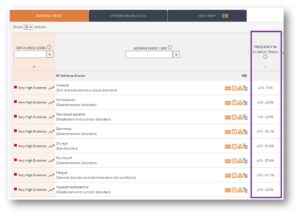
Vaccines are now covered by OFF-X. Safety data of all vaccines approved in the United States and Europe has been manually extracted from the corresponding FDA labels and EMA EPARs, including information about the adjuvants and excipients of each vaccine. These new pieces of data will be leveraged in a forthcoming release of OFF-X as additional criteria when comparing the safety profile of vaccines to assess the potential role of adjuvants or excipients in safety signals. Safety liabilities of all kinds of vaccines, both approved or under development, reported in journal articles, congresses, company communications, clinical trial registries and regulatory communications will be prospectively covered starting on Q1 2023.
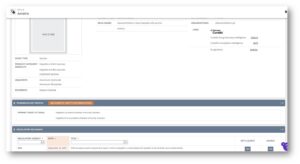
The Secondary Pharmacology Panels analytic tool has been enhanced with the incorporation of the OFF-X Target/Class Score to each target-adverse event association. Leverage this unique, updated daily measure of the evidence linking targets and their toxicities to maintain your secondary pharmacology in vitro panels. Using the most recent safety data available across the wide range of publications covered by OFF-X can help anticipate potential toxicities of your lead candidates before starting preclinical development, based on their off-target activities.
