We are excited to announce the release of a faster, more intuitive, and customizable user interface designed to better serve our users. This initial beta release includes:
Enhanced homepage navigation: On the new homepage a more prominent search bar allows users quick access to the safety information required for their assessments. Safety intelligence extracted from the latest drug approvals, label changes, clinical holds and discontinuations is highlighted.
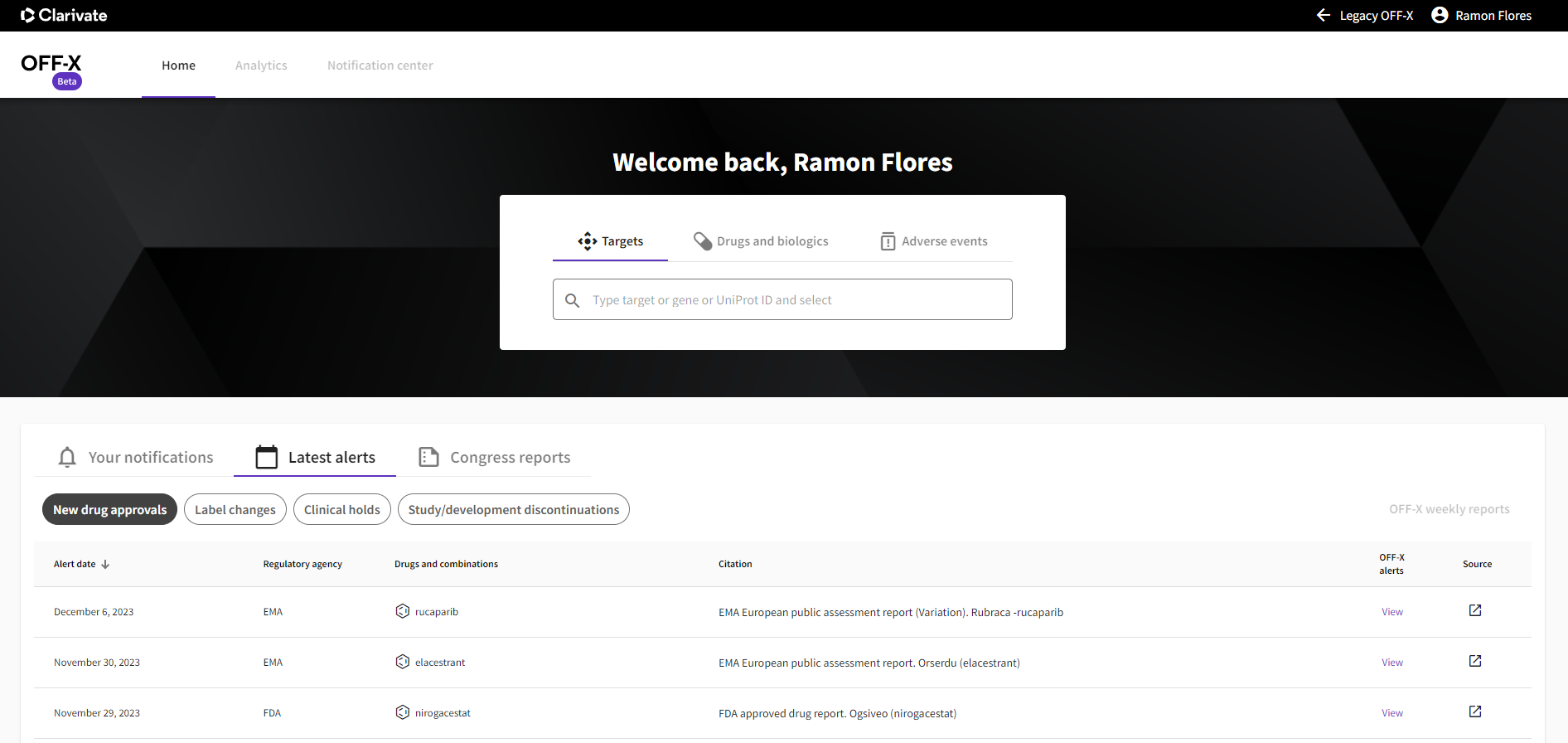
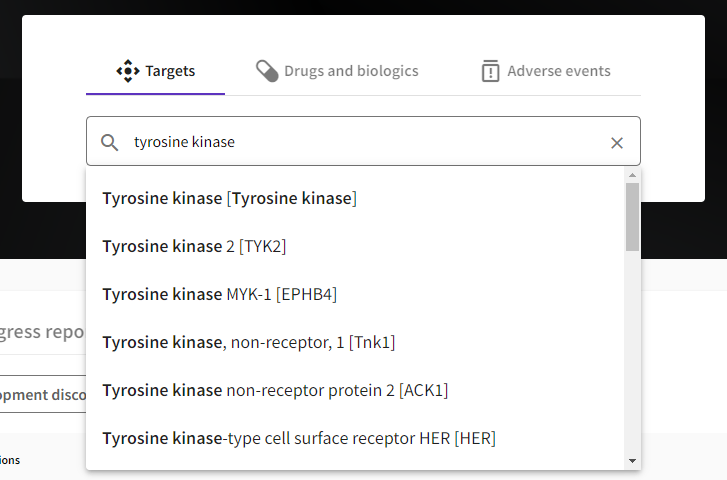
More relevant sorting of search results: The updated search algorithm presents results in a more relevant order, enhancing information retrieval speed.
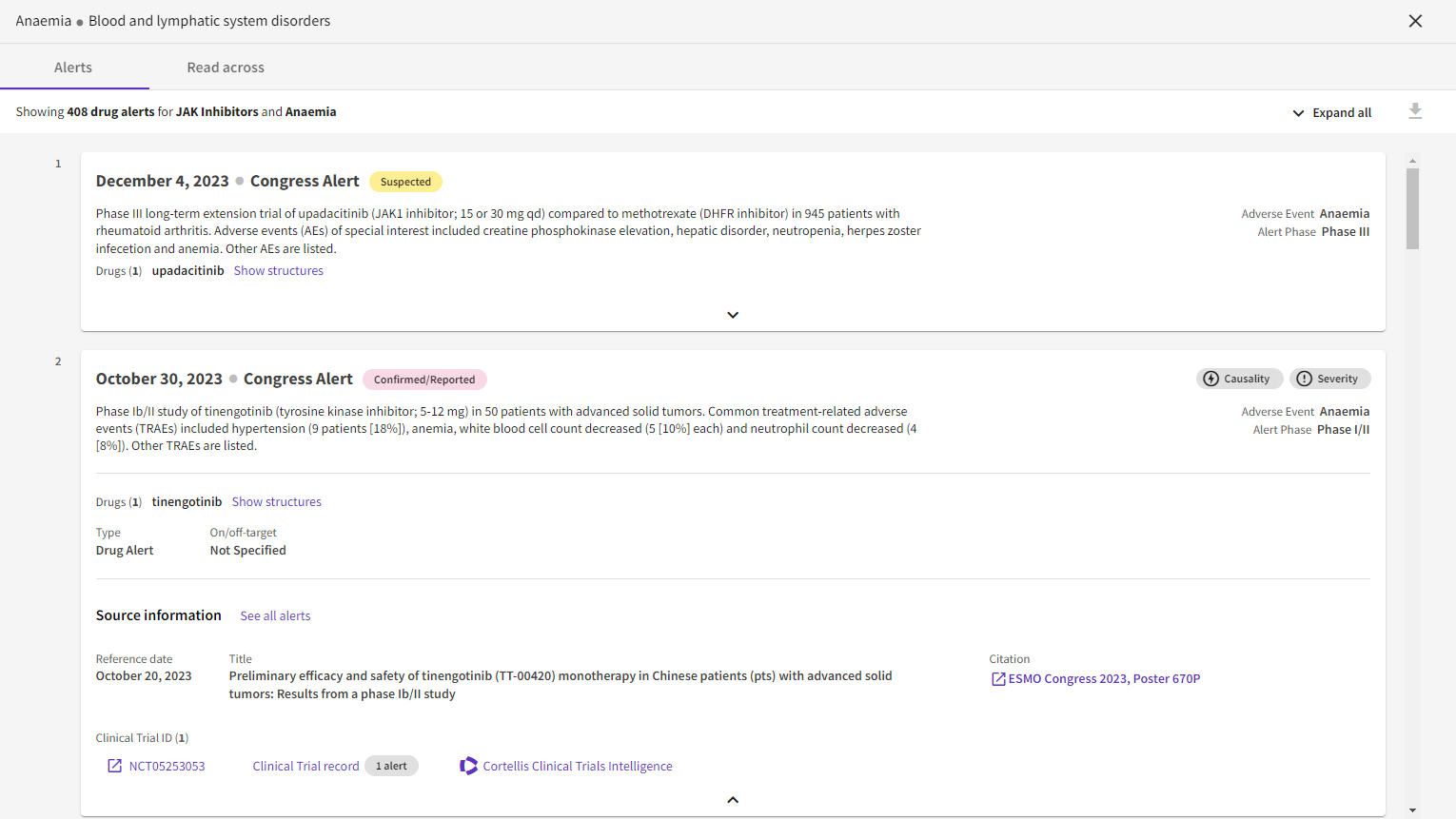
Upgraded safety alerts: Users can now first scan data and then efficiently extract granular details, including causality, severity, and frequency of adverse events from multiple data sources.
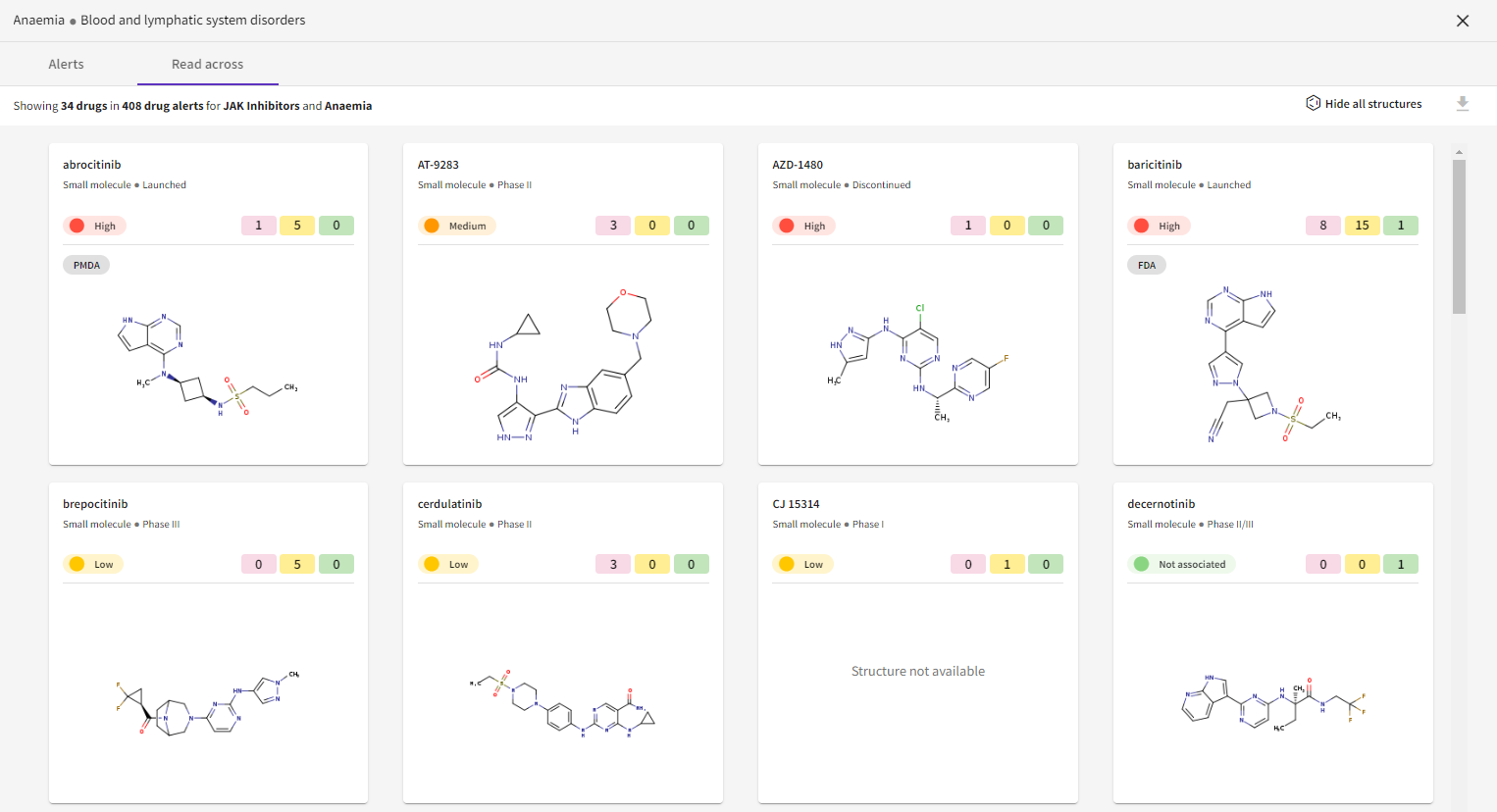
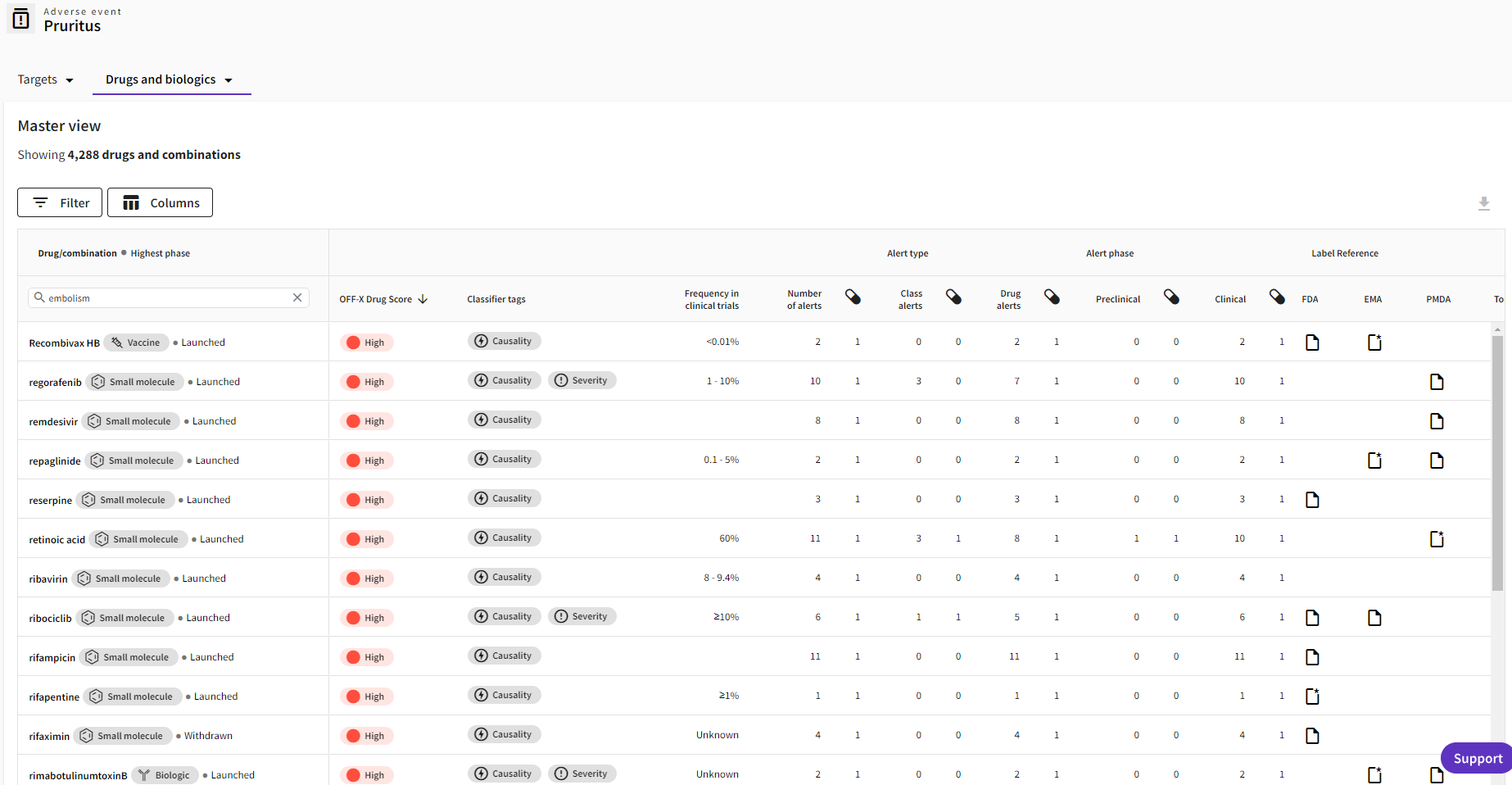
Improved read across view: Information about the OFF-X Drug Score, development phase and drug label have been added to the view that allows users to compare the different drugs associated with a given adverse event.
Enhanced Adverse Event area: Adverse event records have been enhanced following user feedback, to provide details on drug’s development phase, the frequency of adverse events in clinical trials, and information from regulatory approval documents, as well as new filters.
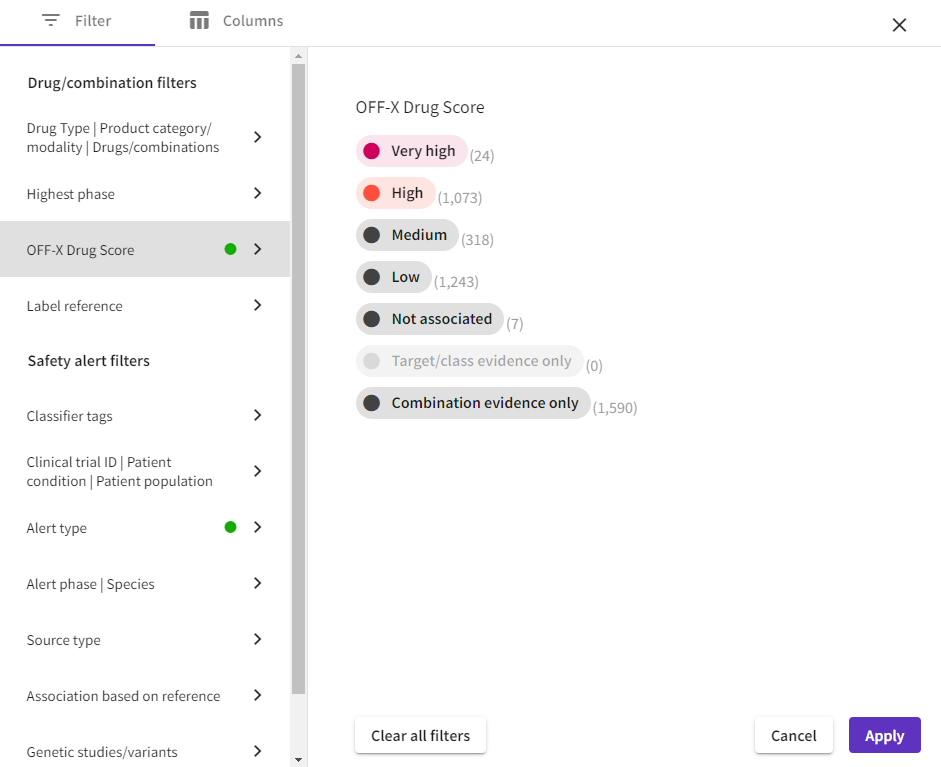
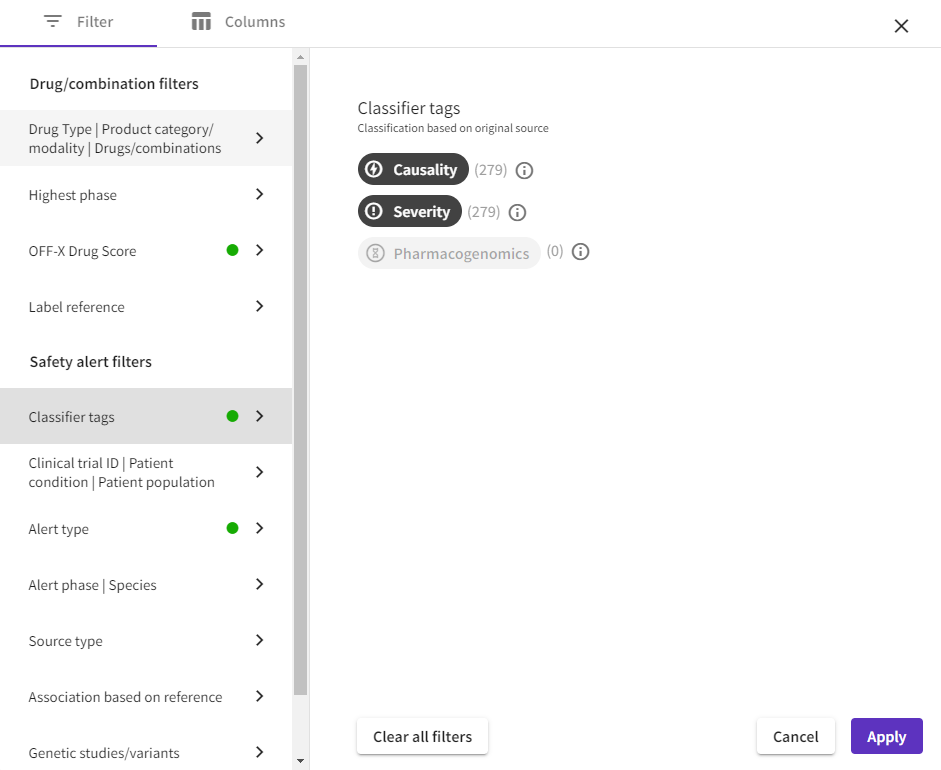
More intuitive filters: The new OFF-X platform empowers users by consolidating all filters into a more intuitive interface, featuring new filtering options. This streamlined experience applies to all tables and visualizations.
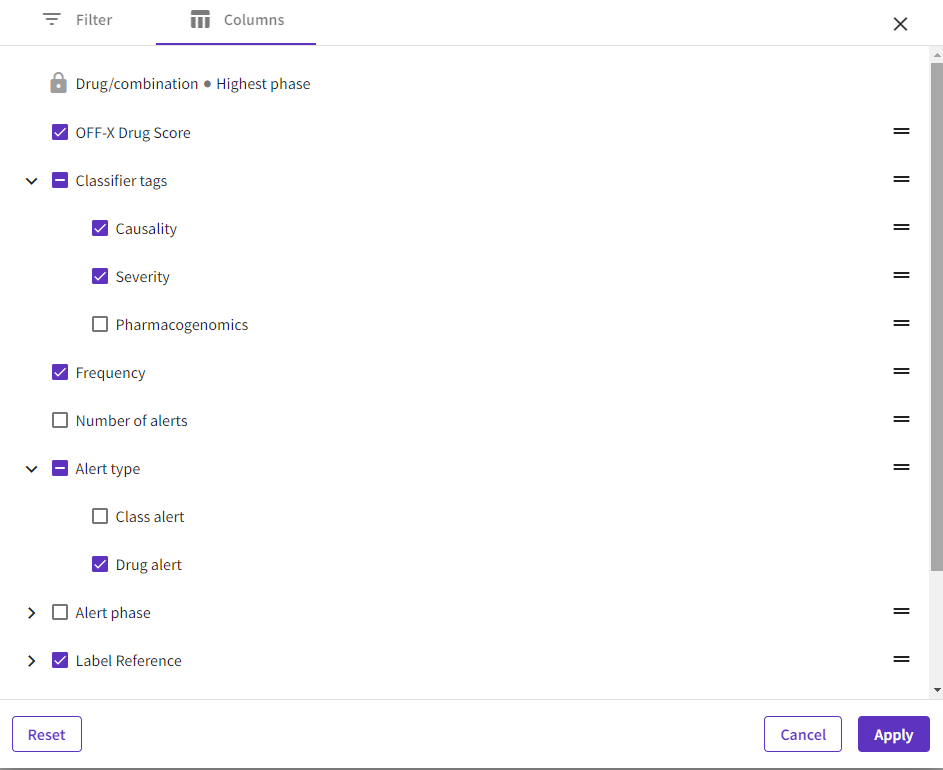
Enhanced customization: New users now have the option to choose the content they want to see in the different tables and visualizations.
More coming soon! We will continue to add new features to the new OFF-X platform in the coming months. Both platforms will coexist until June 2024, when the legacy OFF-X platform will be decommissioned.