About tezepelumab
-
Amgen and AstraZeneca
-
MAb inhibiting thymic stromal lymphopoietin (TSLP)
-
Monthly subcutaneous (SC) injection for treatment of severe asthma
-
30% of patients with severe asthma have a TH2-low phenotype

Tezepelumab is a potential game changer for patients with non-TH2 or TH2-low asthma whose asthma is not well-controlled with inhaled corticosteroids, the current standard of care. Greenlighted by FDA in December and marketed under the brand name Tezspire, it is a first-in-class biologic for this patient population.


Tezepelumab targets the asthma inflammatory process earlier in the pathway than other treatments. It will likely be a first-line biologic for severe TH2-low asthma and a treatment option for TH2-high asthma patients who have failed existing therapies.
September 2018:
For patients with severe asthma without an eosinophilic phenotype:
April 2021:
BLA submitted to the FDA
May 2021:
Filing submitted in Japan
July 2021:
FDA granted priority review
August 2021:
Filing submitted in the EU
December 2021:
FDA authorized use as add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma
Expected launch:
2022: United States
2023: Europe and Japan
Patents estimated to expire beginning in 2028
How will tezepelumab impact the market for asthma?
What gaps in treatment does tezepelumab fill?
Tezepelumab targets a subset of asthma patients who are underserved by available therapies and therefore have high unmet need, including patients with uncontrolled asthma with a non-TH2 /TH2-low phenotype who do not respond well to existing therapies. Dependence on oral corticosteroids for disease control is often not wholly effective and associated with long-term side effects. This is one of the most exciting emerging therapies for asthma treatment.
What hurdles might it need to overcome to reach blockbuster status?
Tezepelumab will face significant competition in TH2-high asthma patients, for whom it is expected to be used as later-line therapy given physician familiarity with existing biologics. With its later-to-market entry than other biologics for this patient population, payer restrictions could constrain uptake, similar to other high-priced biologics. For TH2-low asthma, there is no competition. For both phenotypes, the niche patient population with uncontrolled severe asthma could limit its overall patient share.
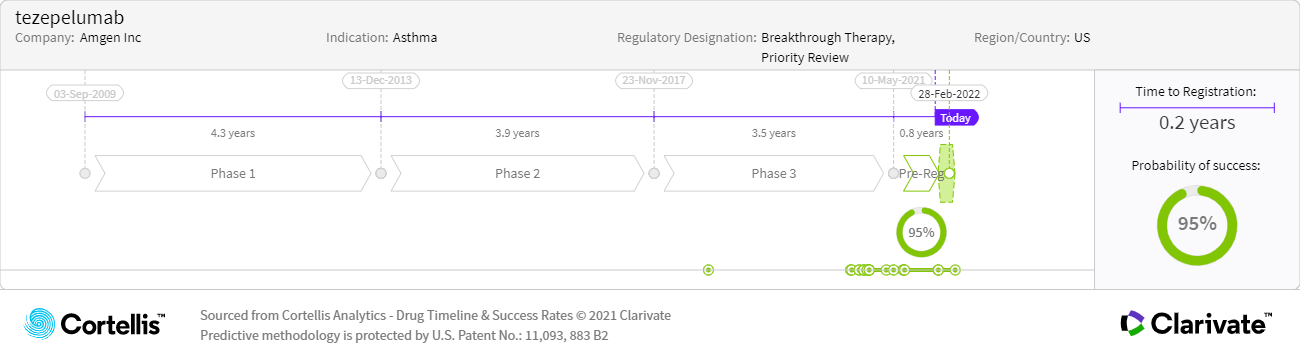
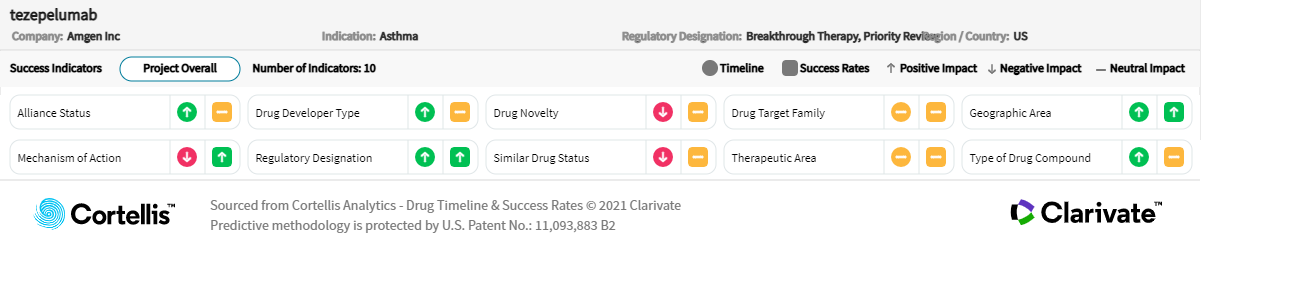
– Source: Cortellis Competitive Intelligence, Drug Timeline & Success Rate prediction current as of December 15, 2021
Access our global intelligence, advanced analytics and global team of experts.