About teplizumab
-
Provention Bio Inc
-
Anti-CD3 monoclonal antibody
-
Daily intravenous administration for 12-14 days to delay progression to clinical T1DM in at-risk people
-
~1.8 million cases of T1DM in the G7 markets in 2021

Teplizumab is the first immunotherapy to launch for T1DM and is a landmark drug given its potential ability to preserve beta cell function and delay the need for insulin treatment.


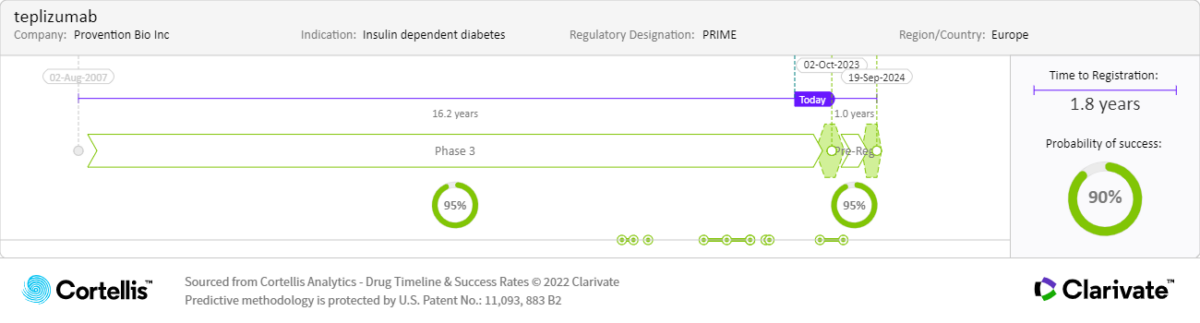
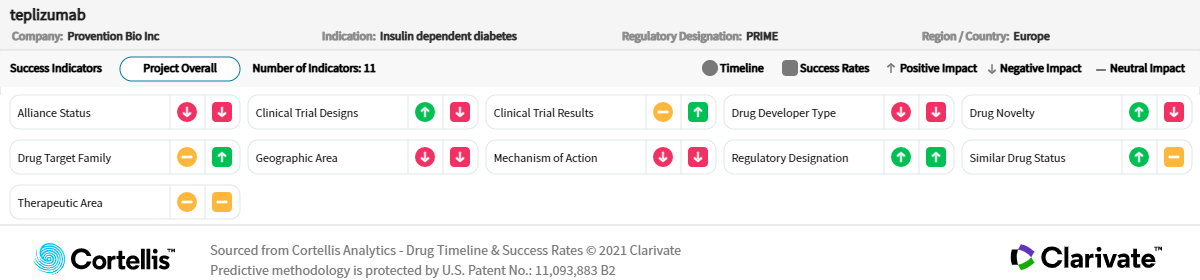
Teplizumab is an Fc receptor–nonbinding anti-CD3 monoclonal antibody that was granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration (FDA) based on results from the phase 2 At-Risk trial in at-risk children and adolescents aged 8 years to 17 years without a diagnosis of T1DM but with a relative with T1DM. In the study, the onset of T1DM was delayed or prevented. The phase 3 PROTECT is assessing the efficacy and safety of teplizumab in children and adolescents aged 8 years to 17 years with a diagnosis in the previous six weeks. Findings from this study will also support the request for additional pharmacokinetic/pharmacodynamic (PK/PD) data in the complete response letter (CRL) from the U.S. FDA in 2021.
October 2019:
• Priority Medicines (PRIME) designation: European Medicines Agency (EMA)
February 2022:
• Biologics License Application (BLA)
November 17, 2022:
• Approved: U.S. FDA
December 2022:
• U.S. launch
Expected launch:
• 2024: Europe
Patents estimated to expire beginning in 2026
Access global intelligence, advanced analytics and global experts from Clarivate.