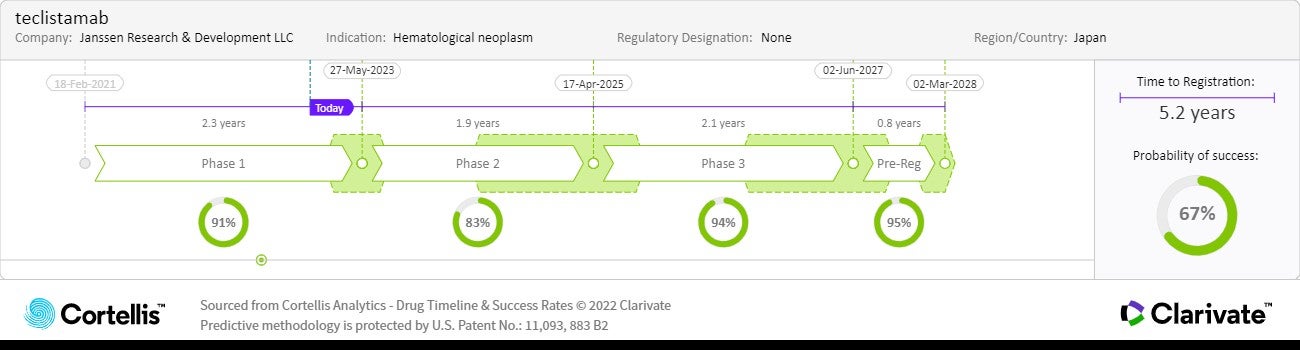
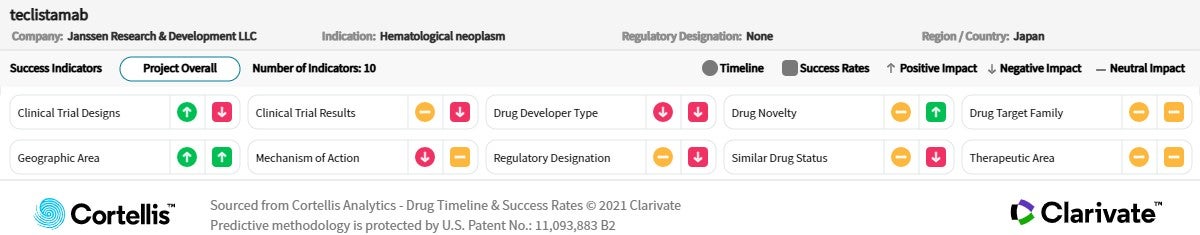
About teclistamab
-
Janssen Pharmaceutical Companies of Johnson & Johnson
-
Bispecific antibody targeted at BCMA and CD3
-
Weekly subcutaneous injection to treat R/R multiple myeloma
-
~72,000 diagnosed incident cases of multiple myeloma in the G7 markets in 2021