About daprodustat
-
GlaxoSmithKline plc.
-
Hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI)
-
Oral administration to treat CKD-related anemia, regardless of dialysis status
-
~67 million cases of CKD in the G7 markets in 2021

Daprodustat belongs to a novel class of oral treatments for CKD-related anemia. Already available for CKD-related anemia in Japan, its uptake has been impressive. In other markets, upon approval, it is expected to differentiate itself from entrenched, injectable erythropoiesis-stimulating agents (ESAs) by its safety profile, efficacy and oral administration. As such, it will help fill the gap in safe treatments for this growing patient population.


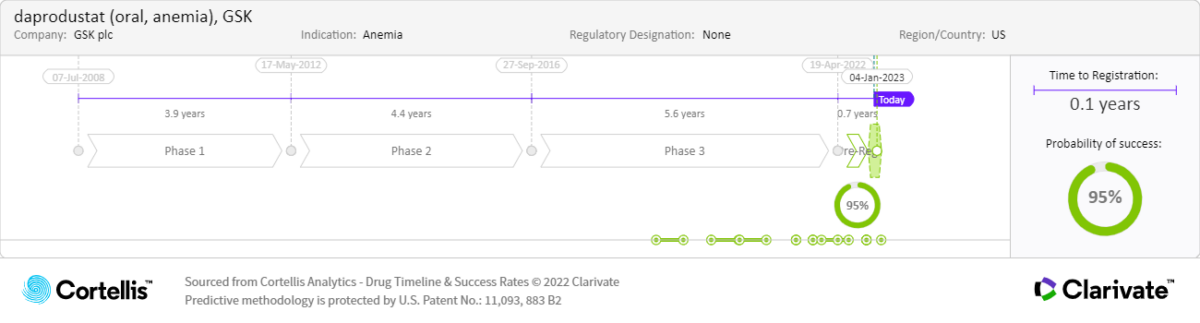
Approved by the Ministry of Health, Labour and Welfare (MHLW) in Japan and under review by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA), daprodustat is an HIF-PHI developed to treat anemia associated with CKD, which has a high incidence rate and few effective, safe treatment options. The New Drug Application (NDA) submitted to the U.S. FDA was supported by results from the phase 3 ASCEND program, which included five studies investigating daprodustat across the spectrum of CKD:
In 2018, GSK plc and Kyowa Hakko Kirin entered into a strategic collaboration agreement for Kyowa Hakko Kirin to commercialize daprodustat in Japan.
March 2022:
• Marketing authorization application (MAA) validated: European Medicines Agency (EMA)
April 2022:
• NDA accepted: U.S. FDA
February 1, 2023:
• PDUFA date
Actual and expected launch:
• 2020: Japan
• 2023: United States and Europe
Patents estimated to expire beginning in 2027
Access global intelligence, advanced analytics and global experts from Clarivate.