Tenofovir Alafenamide
VEMLIDY®

About VEMLIDY®
-
Patented by Gilead Sciences Inc.
-
Anti-HBV reverse transcriptase inhibitor to treat chronic hepatitis B viral (HBV) infection
What to watch?
Will licensing agreements impact the timing of generic entrants? Will the generic availability of VIREAD® impact market performance and interest?
Review and approval status
November 19, 2015
First E.U. approval date
November 10, 2016
First U.S. approval date
December 19, 2021
Japan re-examination period expiry
About VEMLIDY® in the market
-
Under litigation in the United States, with six ANDA filings: Apotex Inc, HEC Pharm Group, Hetero Drugs Limited, Laurus Labs, Lupin Limited and Shilpa Medicare Ltd
-
In May 2023, expiry of the stay of FDA approval concerning the ANDAs in the United States
-
Experienced an increase in global sales by 28.5%, from year ending 2019 to year ending 2020
U.S. market share
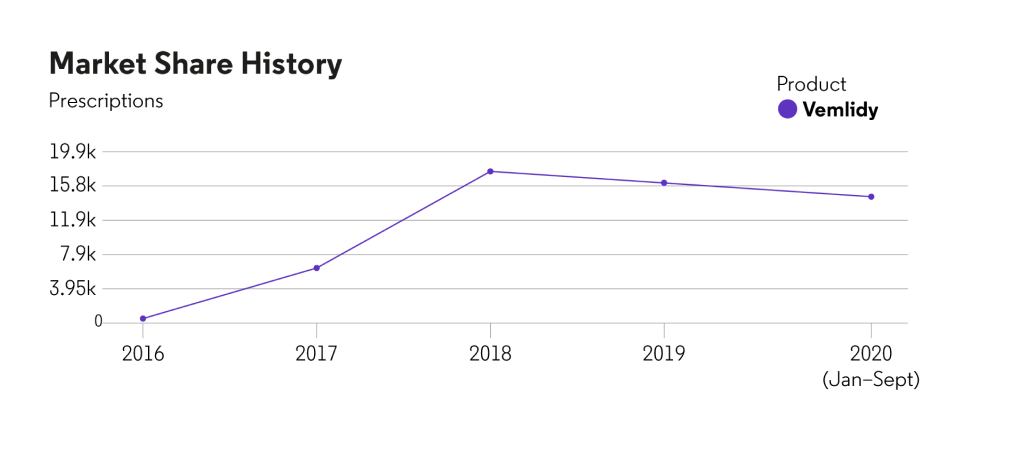

*Source: Cortellis Generics Intelligence; Japan has the highest number of constraining patents
About Gilead Sciences Inc.
-
Gilead commercializes prescription products for several therapeutic areas including HIV/AIDS, liver diseases, serious respiratory and cardiovascular conditions, cancer and inflammation.
-
Marketed products for liver diseases: EPCLUSA® (sofosbuvir, velpatasvir), HARVONI® (ledipasvir, sofosbuvir), HEPSERA® (adefovir dipivoxil), SOVALDI® (sofosbuvir), VIREAD® (tenofovir disproxil fumarate), VOSEVI® (sofosbuvir, velpatasvir, voxilprevir) and VEMLIDY® (tenofovir alafenamide)
-
In the main regulated markets, generic versions only for HEPSERA® and VIREAD®
Patient impact
Patients with a chronic HBV infection experience slow disease progression, and initiation of therapy can be delayed based on patient characteristics and evidence of active viral replication.
However, once on treatment, patients with HBV are often on lifelong daily therapy to maintain viral suppression and prevent the detrimental long-term effects of untreated infections like hepatitis flares and liver damage. When choosing an appropriate treatment, the cost of long-term therapy is an important consideration by physicians, in addition to safety and tolerability.
~2.5M
Drug Timeline & Success Rates



Based on Cortellis data, API is excessively available, primarily from manufacturers in India and Mainland China. Gilead granted non-exclusive rights for a number of companies to manufacture to ensure access to anti-viral medicines to address unmet medical needs.
*Source: Cortellis Generics Intelligence; Data current as of April 14, 2021