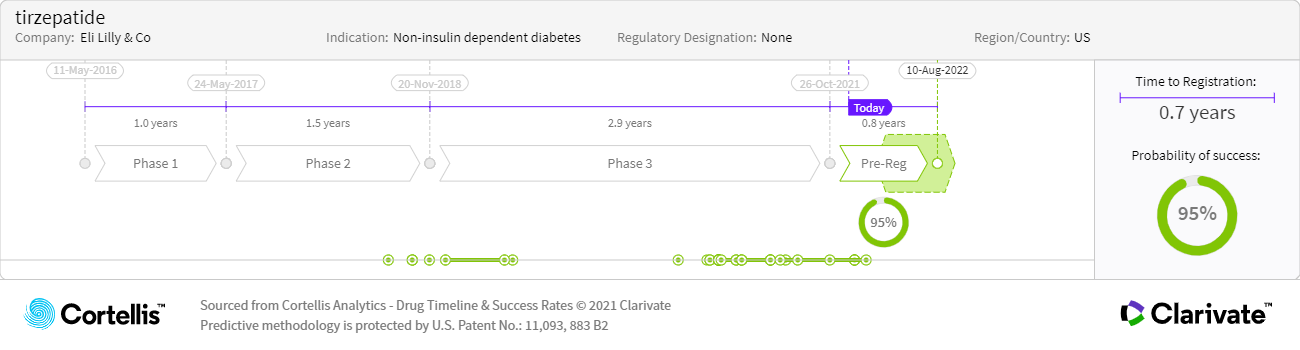
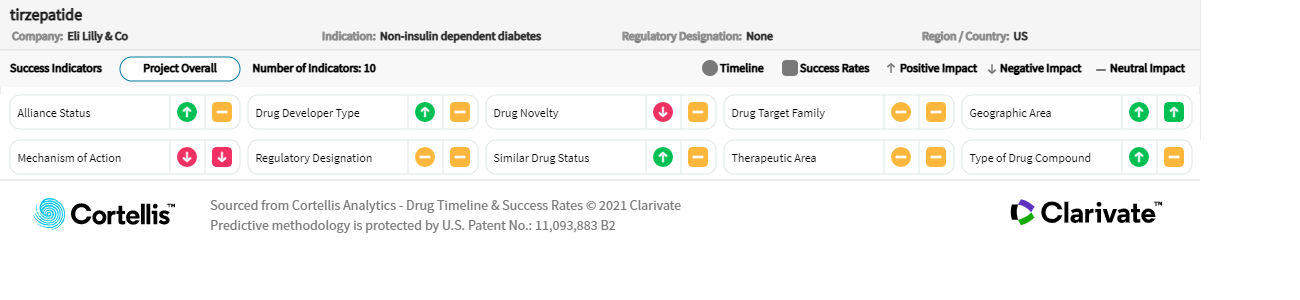
About tirzepatide
-
Eli Lilly and Company
-
GLP-1/gastric inhibitory polypeptide (GIP) receptor agonist
-
Weekly SC administration to treat T2DM
-
Also being studied to treat obesity and non-alcoholic steatohepatitis (NASH)
-
462 million people globally have T2DM
-
1.4% annual increase in drug-treated populations in G7 due to increasing rates of obesity and the aging population