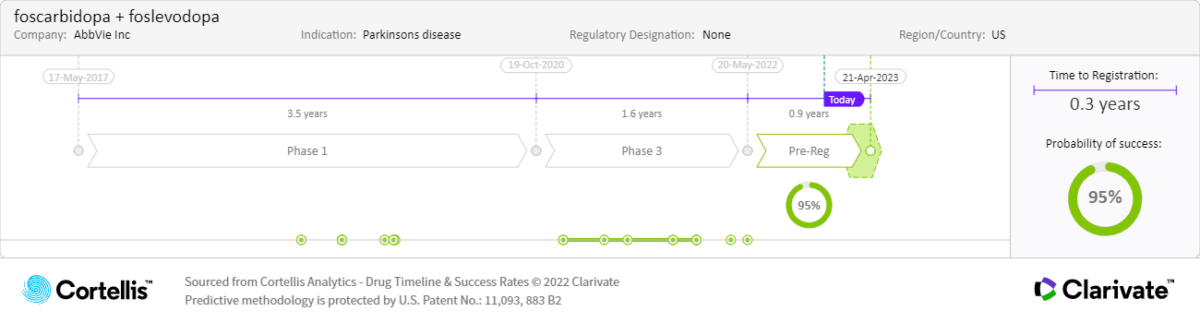
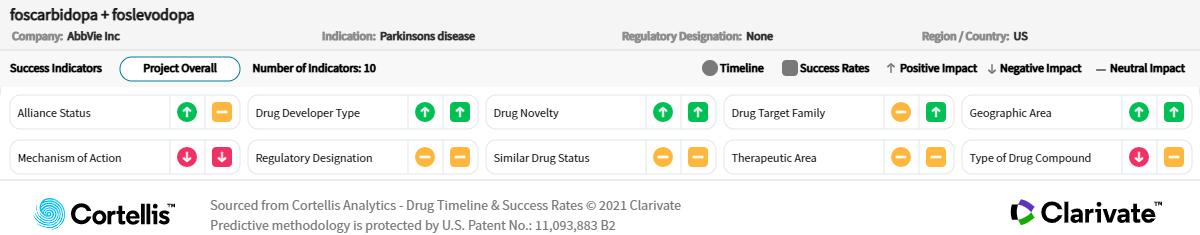
About foscarbidopa/
foslevodopa
-
AbbVie
-
Carbidopa and levodopa prodrugs
-
Continuous 24-hour subcutaneous infusion to treat motor fluctuations in patients with advanced Parkinson’s disease
-
2.8 million diagnosed prevalent cases of Parkinson’s disease in the G7 markets in 2021