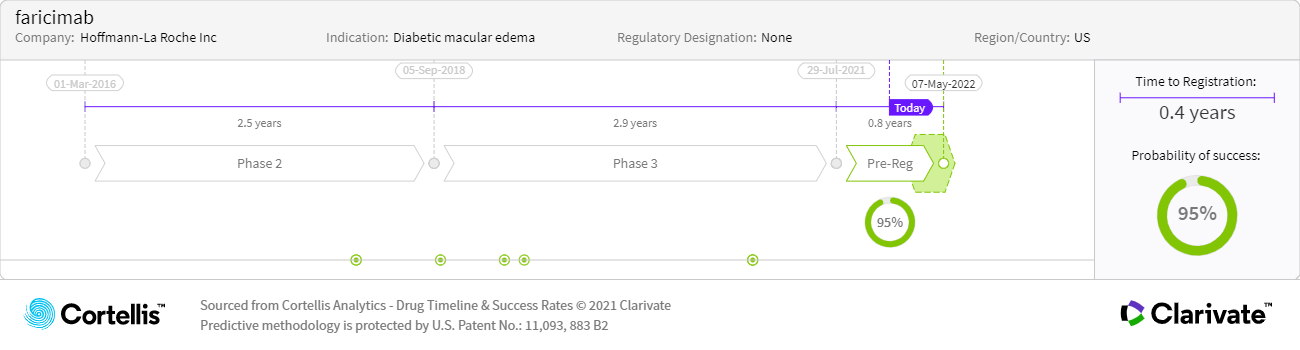
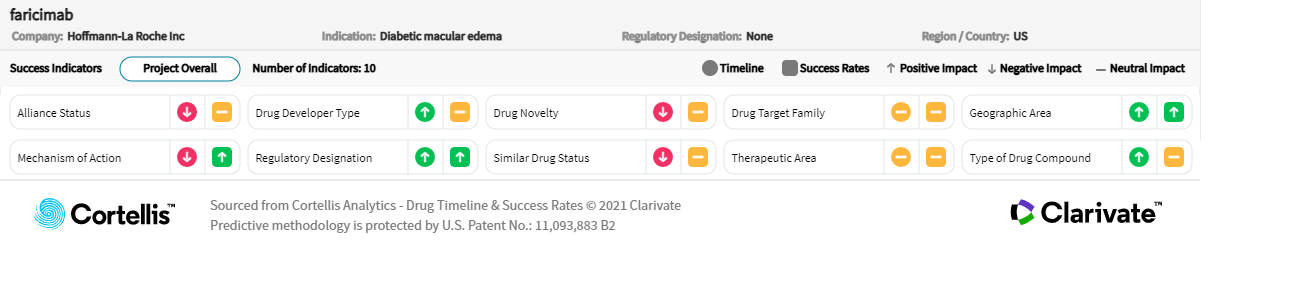
About faricimab
-
Roche and Chugai Pharmaceutical
-
Bispecific VEGF/Ang-2 mAb
-
Intravitreal (IVT)-administered treatment of DME and wet AMD
-
Also being studied to treat retinal vein occlusion (RVO)
-
15% of adults with T2DM have DME
-
3.6M people have wet AMD in the G7 markets