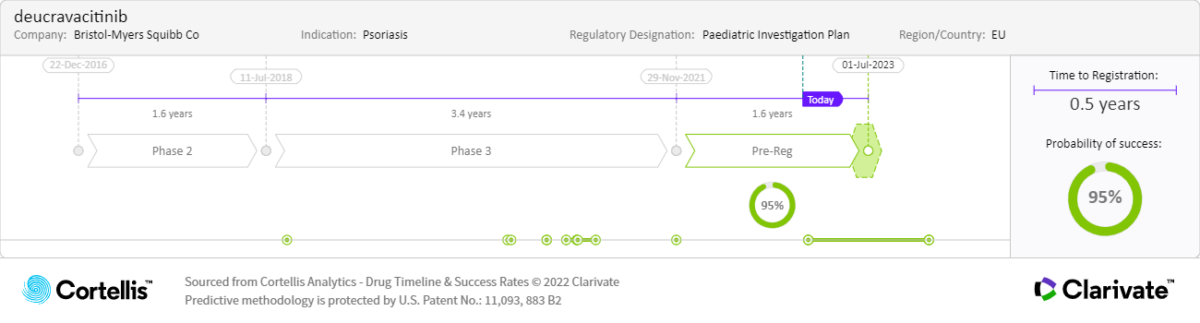
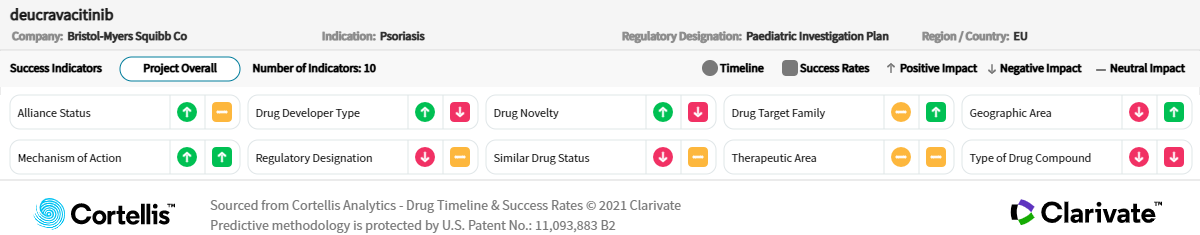
About deucravacitinib
-
Bristol Myers Squibb
-
Allosteric TYK2 inhibitor
-
Once-daily oral administration to treat moderate-to-severe plaque psoriasis
-
Also being investigated to treat pustular psoriasis, erythrodermic psoriasis, psoriatic arthritis, systemic lupus erythematosus and inflammatory bowel disease
-
~11.7 million symptomatic psoriasis cases in the G7 markets in 2021
-
45 years - average age of symptomatic psoriasis cases