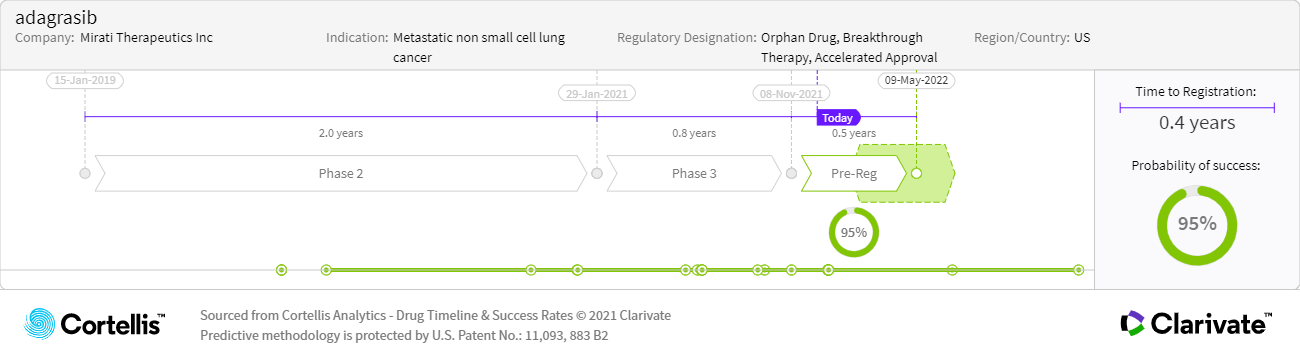
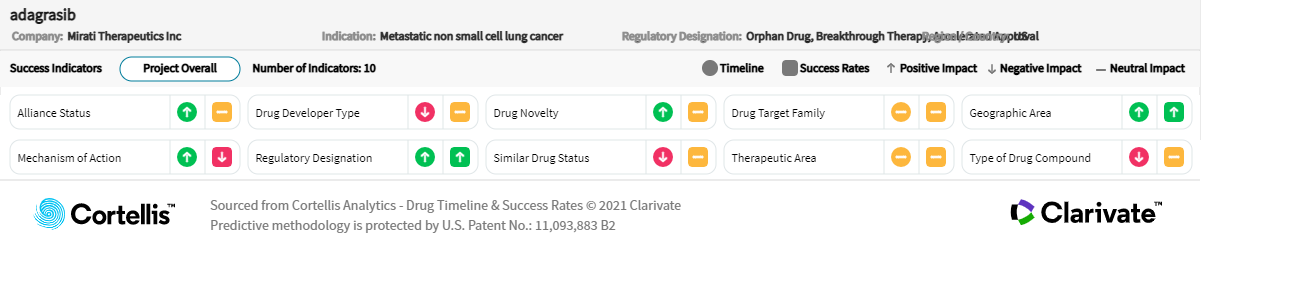
About adagrasib
-
Mirati Therapeutics Inc and Zai Lab Limited
-
KRAS GTPase inhibitor
-
Twice-daily, second-line, oral treatment of advanced KRASG12C-positive solid tumors: metastatic NSCLC and CRC
-
KRASG12C-mutant disease:
• 11-13% of metastatic NSCLC
• 3-4% of metastatic CRC