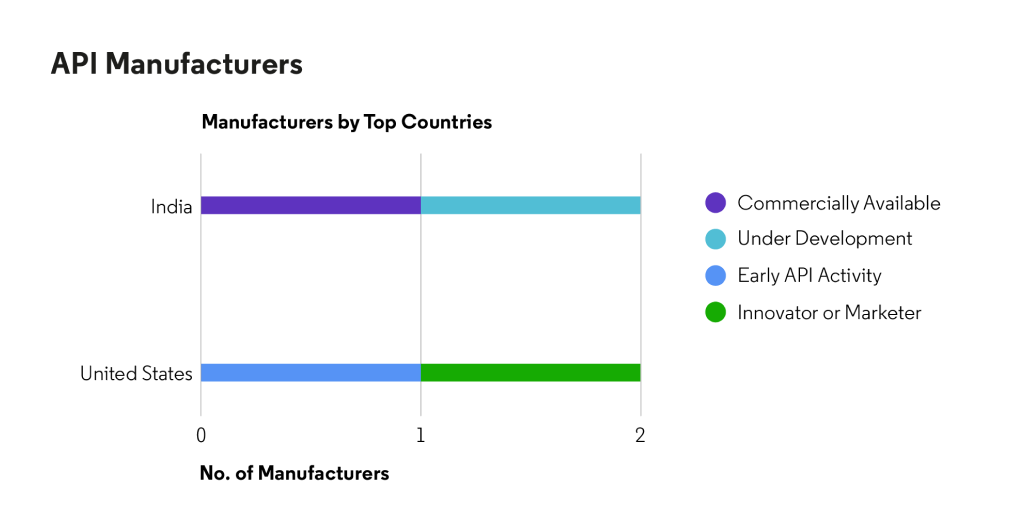
Ponatinib Hydrochloride
ICLUSIG®

About ICLUSIG®
-
Marketed by Takeda Pharmaceutical Company Limited
-
Orally bioavailable Bcr-Abl tyrosine kinase inhibitor
-
Treatment of adult patients with treatment-resistant or intolerant chronic phase (CP) chronic myeloid leukemia (CML), accelerated phase (AP) or blast phase (BP) CML or Philadelphia chromosome–positive (Ph+) CML for which no other kinase inhibitors are indicated, and T315I-positive CML or T315I-positive Ph+ ALL
Review and approval status
December 14, 2012
First U.S. approval
July 1, 2013
First E.U. approval
Actual and expected launch:
- 2026: Patent expiry: United States
- 2027: Patent expiry: Japan
- 2028: SPC expiry: European Union
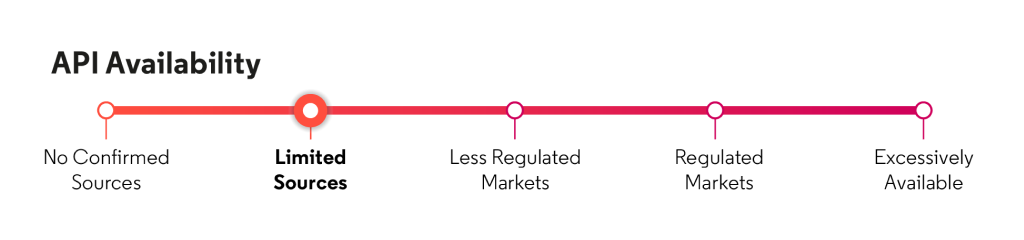
API availability