Adalimumab
HUMIRA®

About HUMIRA®
-
Patented by AbbVie Inc.
-
TNF-α inhibitor to treat many autoimmune conditions with inflammation as a central mechanism, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, plaque psoriasis, juvenile idiopathic arthritis, ulcerative colitis, moderate to severe hidradenitis suppurativa and more
What to watch?
Will the U.S. market share for HUMIRA® trend as it has in the European Union, or will we see significant price erosion with the introduction of numerous biosimilars?
Approvals
December 31, 2002
First U.S. approval date
April 16, 2003
First E.U. approval date
Constraint date forecast:
- U.S. settlement agreements for biosimilar entry: September 30, 2023 -December 15, 2023
About HUMIRA® in the market
-
Experienced an increase in global sales by 10.5%, from year ending 2019 to year ending 2020
-
Several aBLA filings in the United States: Alvotech, Amgen Inc, Boehringer Ingelheim, Coherus BioSciences, Mylan NV, Pfizer Inc, Samsung Bioepis Co Ltd and Sandoz
-
Settlement agreements between AbbVie and several companies regarding adalimumab biosimilar products
-
In the European Union, there are numerous biosimilar products available in the market, although the market share for HUMIRA® still dominant
U.S. market share
Pricing trends in the United States and European Union, for wholesale price based on the average price of all available pack sizes within the selected country
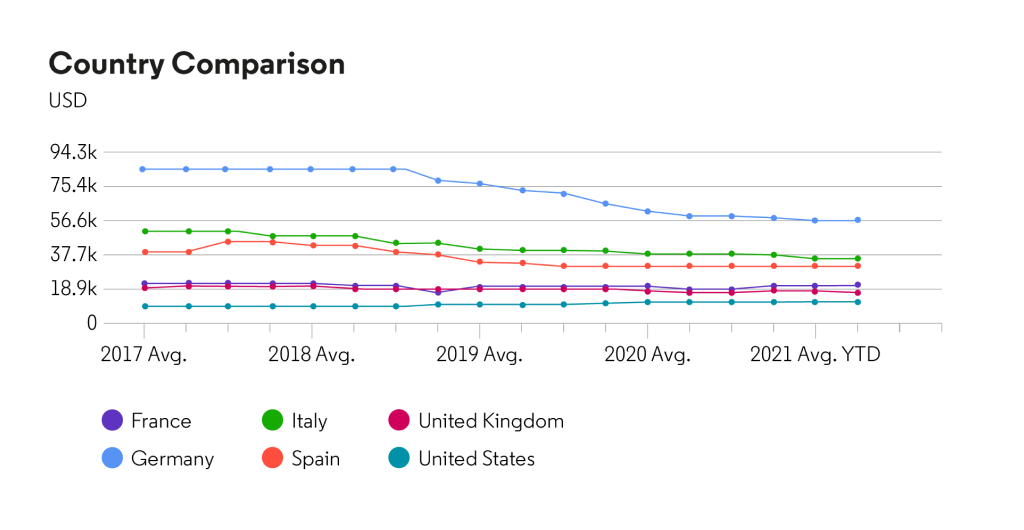
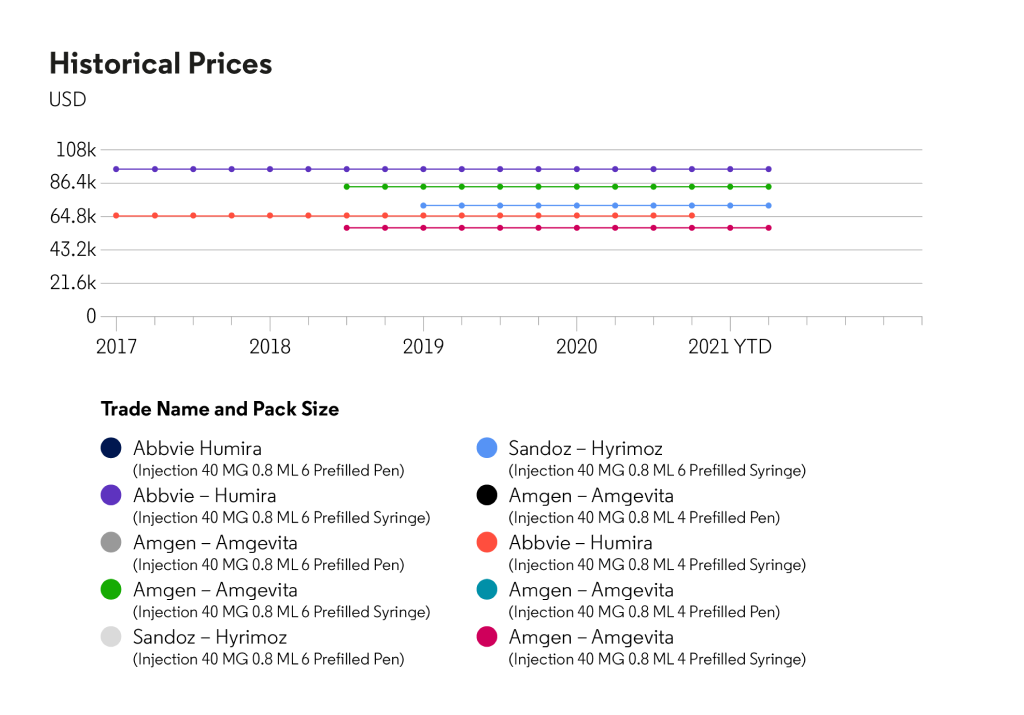
*Source: Cortellis Generics Intelligence
About AbbVie Inc.
-
Portfolio focused on immunology, oncology, neuroscience, virology, eye care and aesthetics
-
Immunology portfolio: HUMIRA® (adalimumab), RINVOQ® (upadacitinib) and SKYRIZI® (risankizumab-rzaa)
-
UPADACITINIB ($629.1 million worldwide in 2020) losing exclusivity in 2029 for major European markets and Japan and in 2031 for the United States
-
Risankizumab-rzaa ($1,625 million worldwide in 2020) losing exclusivity beyond 2030 in all main regulated markets
Patient impact
A generic option could provide a more cost-effective treatment option for the many patients across rheumatology, dermatology and gastroenterology, particularly given the high cost of HUMIRA®.
15-30M
Drug Timeline & Success Rates
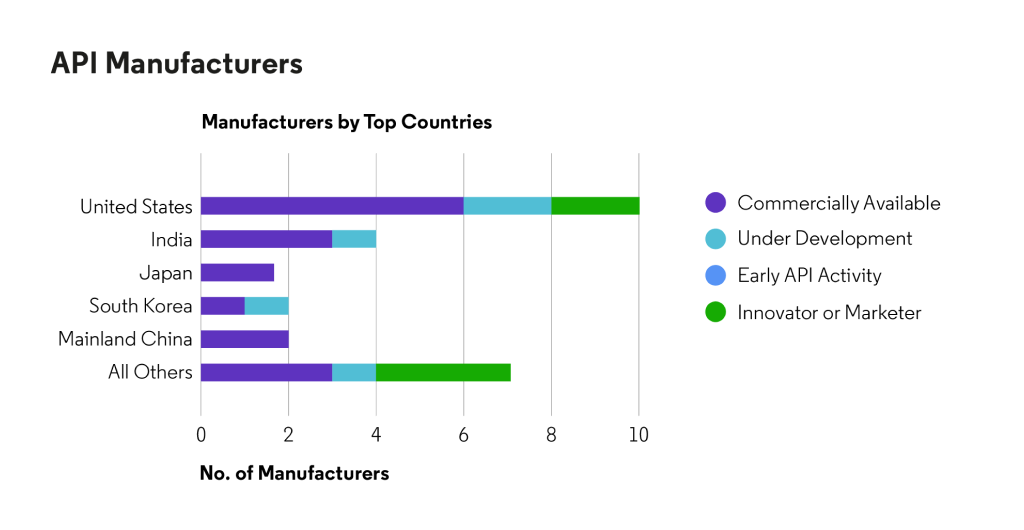
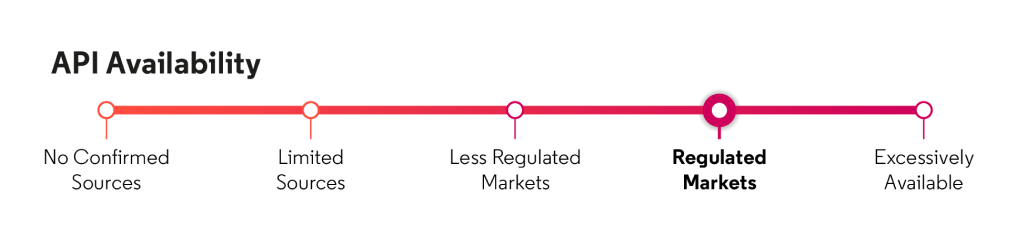
Based on Cortellis data, API is available for regulated markets from manufacturers in the United States and Asia.
*Cortellis Generics Intelligence; Data current as of April 14, 2021