Amid an escalation in the marketing of digital therapeutics (DTx) that diagnose, treat and/or manage health conditions in the United States, the federal government has yet to establish a coverage policy for these software-based interventions for the Medicare population. While some lawmakers seek to change that, United States managed care organizations (MCOs) are moving ahead and covering DTx in commercial plans, particularly for mental health conditions.
As of September 2022, 145 companies have had DTx products in development for a variety of conditions in the United States, according to Cortellis Digital Health Intelligence™. While the insurance industry is trying to keep pace with the steady stream of applications entering the market, lawmakers have introduced bills in the House and Senate (H.R. 7051 and S.R. 3791) directing the Centers for Medicare & Medicaid Services to set up a Medicare payment methodology for DTx.
That hasn’t kept many managed care organizations (MCOs) from making DTx products and services accessible to members with mental health conditions, according to the report Digital therapeutics in mental health: major depressive disorder (MDD), insomnia and opioid addiction, published by Clarivate in June 2022.
As part of the report, the Clarivate U.S. Access & Reimbursement team surveyed pharmacy and medical directors from 40 MCOs across the United States, as well as 102 U.S. psychiatrists. The report indicates that progress is being made in covering DTx that target major depressive disorder (MDD), insomnia and/or opioid addiction. Of surveyed MCOs that cover or plan to cover DTx apps for the three conditions, more than 80% of respondents reported reimbursing DTx for at least one of the conditions in their fully insured commercial plans as of March 2022, with the remainder expecting to do so by 2024. Payers also noted some coverage in their Medicare and Medicaid plans. Nearly two-thirds of MCOs developed a comprehensive coverage policy for DTx, while the rest are working on or intend to develop one.
Payers and providers embrace evidence-based DTx
Half of the psychiatrists surveyed reported having prescribed or recommended DTx to patients within the three studied indications, while the remainder indicated they expect or are open to prescribing them. Survey results suggest that DTx uptake will improve as more psychiatrists become aware of the apps and insurers smooth the path to access.
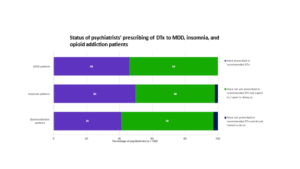
Source: Clarivate, Digital therapeutics in mental health: major depressive disorder (MDD), insomnia and opioid addiction
Rather than creating a new digital benefit for DTx, surveyed MCO directors indicated that coverage is commonly handled through existing benefit structures and that DTx are frequently covered through both the pharmacy and medical benefits—the latter of which became more feasible after CMS established a new HCPCS Level II billing code for digital behavioral therapies in April 2022. Additionally, Clarivate research indicates that most pharmacy benefit plans will have a separate DTx formulary by 2024.
The report also examined reimbursement and uptake of four apps listed by the Digital Therapeutics Alliance for the three conditions: Pear Therapeutics’ reSET-O for opioid addiction and Somryst for insomnia, Orexo’s Deprexis for MDD and Big Health’s Sleepio for insomnia. Psychiatrists were also surveyed about a free app for MDD, Limbix’s SparkRx.
What the apps have in common is that they provide virtual sessions that lead patients through several weeks of cognitive behavioral therapy designed to improve sleep, lessen depression or curb opioid abuse. Interestingly, more than half of surveyed psychiatrists agreed that a digital therapeutic could potentially replace drug therapy in some patients.
The FDA allowed DTx products for psychiatric disorders to be marketed as class II medical devices without FDA clearance during the public health emergency declared for the COVID-19 pandemic, which was recently extended to run through the remainder of 2022.
As a result, few products have FDA clearance. One exception is Pear Therapeutics, which has not only secured FDA clearance but also required a prescription for its reSET-O and Somryst apps. The survey revealed that reSET-O was one of the most frequently covered apps by surveyed MCOs (48%) and the most frequently prescribed by surveyed psychiatrists (44%). Of the studied apps, reSET-O was the only one paired with a drug therapy, buprenorphine.

Source: Clarivate, Digital therapeutics in mental health: major depressive disorder (MDD), insomnia and opioid addiction
Five key considerations for driving coverage and uptake of DTx
With multiple DTx entering the mental health space, developers are tasked with distinguishing their products to payers and providers. Among the key takeaways gleaned from the report:
- Companies seeking DTx coverage in fully insured commercial plans will likely have to contract with payers for formulary access. Payers indicated rebate contracting is common and suggested that outcomes-based contracting will be a likely approach to reimbursement.
- While FDA clearance is not essential to coverage, it will help to distinguish DTx apps from numerous competitors seeking formulary or medical benefit coverage.
- Determining the clinical effectiveness of DTx is viewed as a major challenge by MCO directors. Payers and psychiatrists are most receptive to apps that demonstrate efficacy though randomized clinical trials data and/or real-world evidence.
- Payers and providers value mental health apps that demonstrate sustained efficacy for months after a program ends, particularly in opioid addiction, where ongoing abstention is a valued outcome.
- Most psychiatrists reported that a DTx program will be at an advantage if paired with a drug therapy, particularly in opioid addiction.
What’s next?
The recent extension of the COVID-19 public health emergency allows DTx to be marketed for psychiatric disorders without FDA clearance, at least through 2022.
If Congress passes the Access to Prescription Digital Therapeutics Act, the market would be reinvigorated with the establishment of a payment methodology for Medicare reimbursement. Identical bills were introduced in both houses in March and referred to committee.
To learn more about how to access the full report, or for further information on Clarivate Access and Reimbursement reports, Cortellis Digital Health Intelligence™and other Clarivate solutions, please contact us at HealthCare.support@clarivate.com.
About the author
Chris Lewis is responsible for the coordination, content review, and content generation of U.S. Access & Reimbursement reports at Clarivate, including authoring select A&R reports on managed care trends. Before this position, Ms. Lewis was a senior analyst at HealthLeaders-InterStudy. She also launched and authored the Pharmacy Benefit Manager profile series.





