Reimbursement in South Korea depends upon a favorable review by Health Insurance Review Agency (HIRA), and some therapies may not be reimbursed because of shortcomings from the perspective of HIRA.
To help pharma companies plan clinical trials and economic evidence submission in order to achieve favorable access in South Korea, we used the Context Matters Market Access Platform to analyze the clinical and economic rationales behind HIRA’s decisions for 15 oncology indications over the past 5 years (2015 – 2019):
4 in 5 decisions valid adequacy of benefit
HIRA benefit assessment decisions in South Korea, from HTA reports across 15 oncology indications, 2015-2019
The vast majority of decisions received valid adequacy of benefit. In a few cases, decisions were considered non-benefit which means that the drugs are not eligible for price negotiations in South Korea.
Source: Context Matters’ Market Access Platform, Accessed March 8, 2020, Decision Resources Group
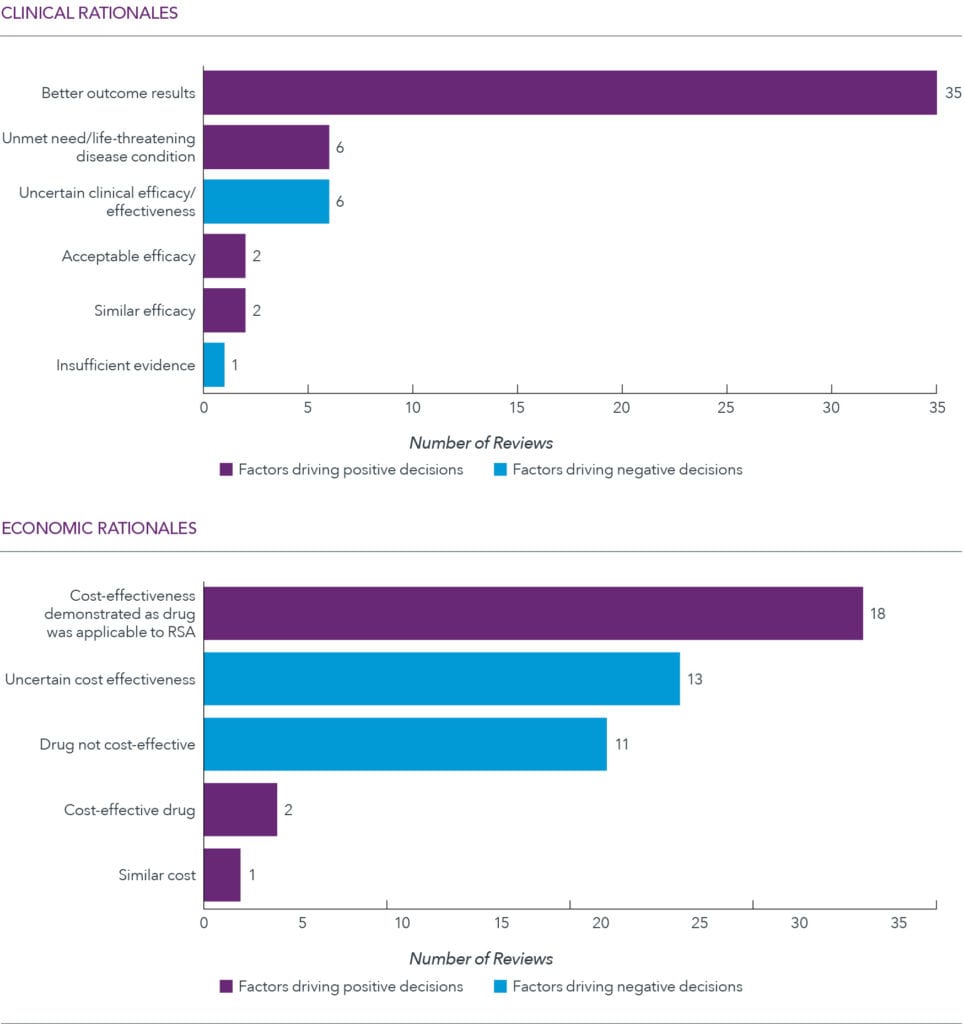
Favorable reimbursement tied to improved efficacy and acceptable cost-effectiveness
Key clinical and economic rationales behind agency’s decision, HTA reports across 15 oncology indications, 2015-2019
Source: Context Matters’ Market Access Platform, Accessed March 8, 2020, Decision Resources Group
Across decisions, favorable reimbursement was tied to improved efficacy and acceptable cost-effectiveness (commonly through a risk-sharing agreement). Direct cost-comparison was the most frequently used economic model. Some medicinal products under review, registered in > 3 foreign countries, were classified as drugs that can omit the submission of economic evaluation documents.
However, some therapies that cleared the efficacy hurdle fell short in terms of demonstrated cost-effectiveness, leading to unfavorable decisions on the part of HIRA. HIRA does not have a formal cost-effectiveness threshold, but implicitly, the threshold is approximately KRW 25 million for all drugs (US$22,000) and KRW 50 million for oncology or rare drugs.
What does this mean for pharma companies assessing this market?
The research indicates that oncology product manufacturers should provide robust clinical data to show better clinical outcome results and strong economic data to demonstrate drug cost-effectiveness to gain market access in South Korea.
Risk-sharing agreements and economic evaluation exemptions improve patient accessibility for drugs without alternatives in life-threatening disease conditions. Uncertainties in clinical efficacy/effectiveness and cost-effectiveness are the key factors correlated with non-benefit.
Understanding the evidence requirement by the reimbursement agency for clinical and economic decision-making could possibly result in quicker patient access to innovative treatments. South Korea is planning to introduce a post-list review focusing on anticancer and orphan drugs.