A new study by the Centre for Innovation in Regulatory Science (CIRS) has shown that despite regulatory convergence over the last 20 years, there are still differences in approval times across global regulatory agencies. The following article summarizes key findings from the latest CIRS R&D Briefing, New drug approvals in six major authorities 2011-2020.
Three major trends came to light from an analysis of review approval times for the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA), the Japan Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada, Swissmedic and the Australian Therapeutic Goods Administration (TGA).
1. Regulatory approval times still differ
Despite regulatory convergence over the last 20 years, the study showed that there were still differences in approval times across the six agencies in 2020 (Figure 1). This difference was particularly apparent for European regulators (EMA and Swissmedic) compared to the other four agencies.
However, there was less variation between the six agencies when comparing the time from submission to the end of scientific assessment (number in parentheses, Figure 1). This highlights that, at least for some agencies, there are activities that occur following the end of scientific assessment, such as administrative activities or additional labelling negotiations with the sponsor.
Figure 1: New Active Substance (NAS) median approval time for six regulatory authorities in 2011-2020
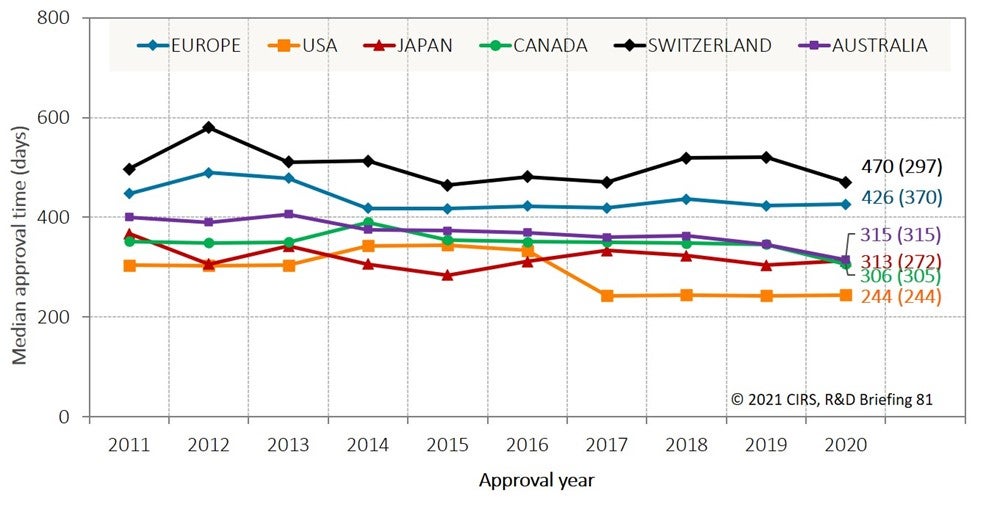
Source: Centre for Innovation in Regulatory Science (CIRS)
Approval time is calculated from the date of submission to the date of approval by the agency. This time includes agency and company time. EMA approval time includes the EU Commission time. N1 = median approval time for products approved in 2020; (N2) = median time from submission to the end of scientific assessment for products approved in 2020.
2. FDA continues to approve the most NASs
The study found that the number of NASs approved by the six agencies has generally increased over the last decade. However, when only the last five years were considered, there was a plateau in approvals by all agencies except the FDA, which continued to approve an increasing number of products. This may be due to the availability of facilitated regulatory pathways at the FDA, or that some medicines approved by FDA, particularly from smaller companies, do not become internationalized.
3. Increasing importance of work sharing
Several regulatory agencies have developed or are developing frameworks for work sharing, helping to avoid duplication of regulatory assessment and encouraging efficient use of resources. As part of the work sharing process, the agencies involved review different parts of the submitted dossier. Although the review is shared, each regulator makes an independent decision regarding approval of the new medicine.
This CIRS study took a closer look at the outcomes of two work sharing initiatives featuring some of the six agencies: the Access Consortium and Project Orbis. Between 2018 and 2020, seven NASs were approved by one or more of the agencies participating in the Access Consortium. Since its initiation in 2019, Project Orbis has facilitated the approval of three NASs (Figure 2). Interestingly, the NASs differed in terms of the orphan designation as well as the type of review undertaken (expedited vs. standard), which highlights differences in agency criteria to obtain expedited review or orphan designation, processes available (Health Canada does not currently have an orphan policy) as well as company strategy to apply for the pathway or designation.
Figure 2: Submission lag and approval times for NASs approved by Project Orbis members in 2020
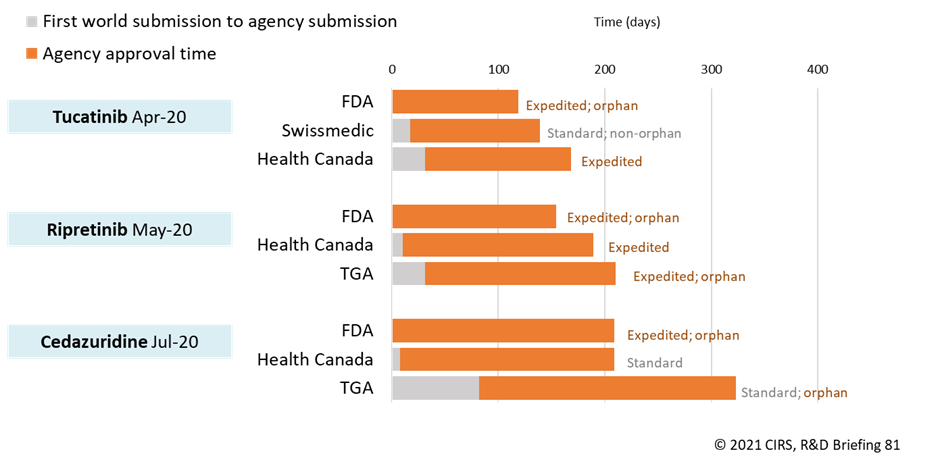
Source: Centre for Innovation in Regulatory Science (CIRS)
Submission gap is calculated as the time from date of submission at the first regulatory agency to the date of regulatory submission to the target agency. ‘Expedited review’ refers to Health Canada/TGA ‘Priority Review’ and Swissmedic ‘Fast Track’. Health Canada does not currently have an orphan policy. Approval time is calculated from the date of submission to the date of approval by the agency.
Impact of COVID-19
The availability and use of expedited as well as other facilitated regulatory pathways is key to addressing areas of unmet need and other public health emergencies such as COVID-19 (see CIRS R&D Briefing 75 for more information). In 2020, COVID-19 therapeutic remdesivir was one of the fastest anti-infective approvals across all six agencies[1], with various expedited and facilitated regulatory pathways being utilized such as conditional approval, FDA Fast Track and the use of rolling review.
Although new ways of working have been identified, the COVID-19 pandemic continues to put enormous pressure on both pharmaceutical companies and regulatory agencies (see CIRS R&D Briefing 80 for more information). CIRS analyses in the coming years may demonstrate the impact of the pandemic on NAS approvals by the six agencies, for example, whether there has been an increase in approval times or a decrease in the internationalization of medicines.
CIRS has been benchmarking regulatory agencies since 2002 using a methodology developed with agencies that ensures like-for-like comparisons. The resulting analyses, published annually since 2012, give unique insights into regulatory processes and practices, identify where improvements can be made and inform company and agency strategies.
For more regulatory insights, download the full briefing, New drug approvals in six major authorities 2011-2020.
[1] Note: vaccines were out-of-scope for this study.





