This week marks the first International NASH Day, an initiative designed to increase both physicians’ and the general population’s awareness of nonalcoholic steatohepatitis (NASH) and to try and address the current unmet needs associated with the disease.
Despite increasing focus on NASH treatment development from the pharmaceutical industry, there is still a clear unmet need for non-invasive diagnostics for the disease. Liver biopsy is currently the only reliable way to officially diagnose and monitor NASH, and is an invasive and expensive procedure for patients – costing up to $3500 in the United States.
The lack of a non-invasive diagnostic is hindering efforts to identify and treat at risk patients, with physicians surveyed by Decision Resources Group citing the invasiveness of a biopsy and patient unwillingness as major factors constraining the use of biopsies in the diagnosis of NASH. A method to accurately quantify and monitor fibrosis in NASH patients is especially important, as fibrosis, above all other histological feature of NASH, appears to be the best determinant of overall survival and liver-related outcomes.1
In order to support NASH diagnosis and getting patients on a path to care, in addition to decreasing the invasiveness and cost to patients, numerous companies, physicians, and academics, are working on novel non-invasive diagnostics.
Non-invasive NASH diagnostics can broadly be categorized into the following categories:
Serum biomarker tests
Simple serum biomarkers are not accurate or reliable enough to use alone as a diagnostic tool. However, tests incorporating several different measures to increase accuracy and reliability have emerged and are increasingly being adopted into practice.
Consortiums such as the EU’s LITMUS (Liver Investigation: Testing Marker Utility in Steatohepatitis), comprising 47 international leading international universities and pharmaceutical companies, and U.S. based NIMBLE (Non-Invasive bioMarkers of metaBoLic diseasE) aim to develop and validate better biomarkers for the diagnosis of NASH/NAFLD.
In addition to developing elafibranor for NASH, Genfit (themselves part of LITMUS) is also developing their own diagnostics, based on micro-RNA biomarkers (especially miRNA-34a and miRNA-122); development of a proprietary companion diagnostic alongside elafibranor would put Genfit in a strong position in terms of increasing diagnosis rates, speeding up recruitment in future trials and likely increasing the popularity of elafibranor with both physicians and payers.
Galmed Pharmaceuticals have partnered with OWL genomics, to develop a companion diagnostic to Galmed’s Phase IIb compound, aramchol, with the aim of predicting therapeutic responses to the drug (a factor which would likely be a positive in the eyes of payers).
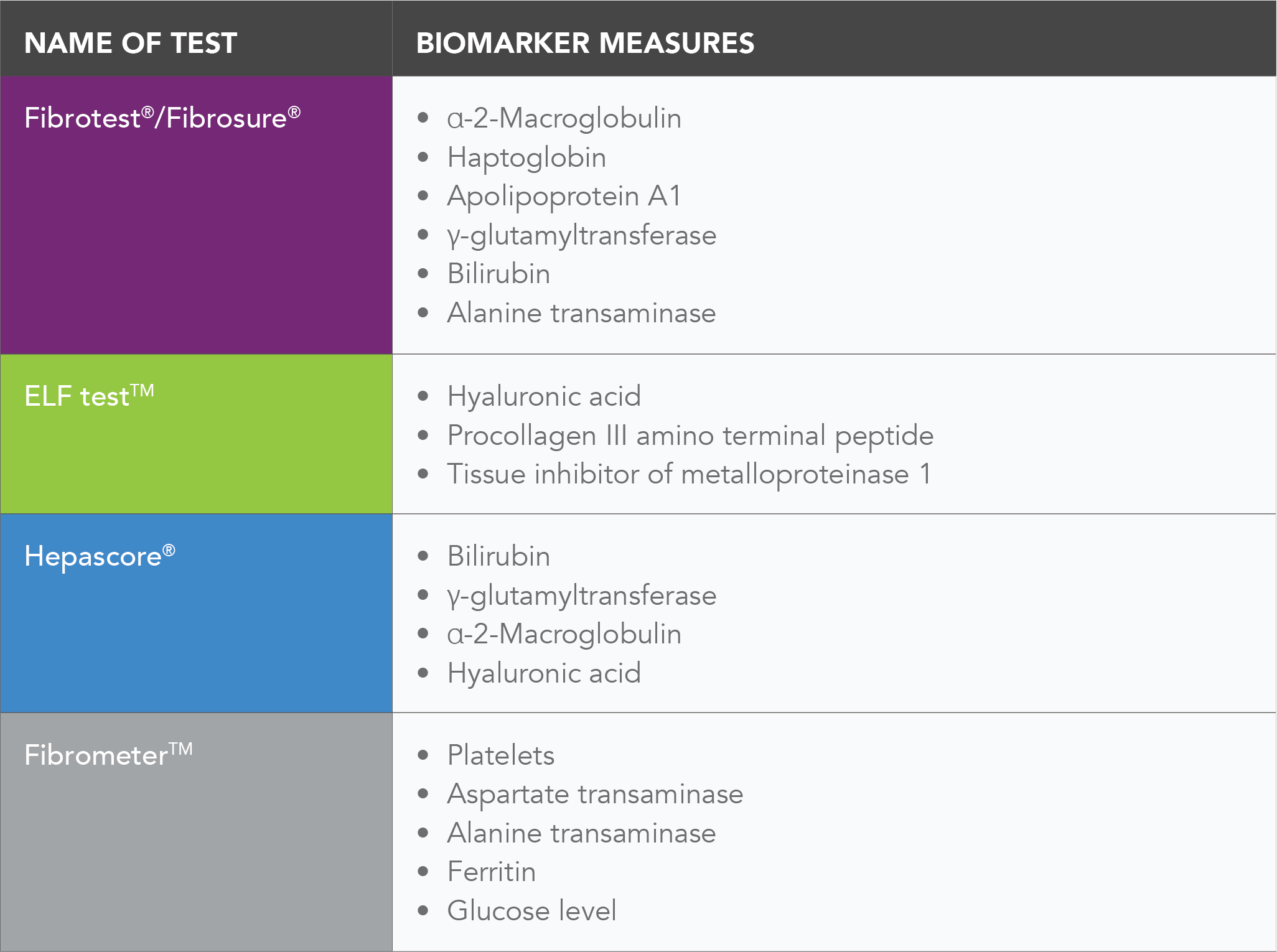
The table below gives examples of a number of commercially available serums tests used to monitor liver health (namely in the form of fibrosis) of NASH patients:
Imaging tests
Numerous imaging modalities exist to aid the diagnosis and monitoring of NASH patients (e.g. simple ultrasound, magnetic resonance imaging, ultrasound elastography, and magnetic resonance elastography).
On paper, an imaging test for NASH could be the perfect solution to invasive diagnosis via biopsy: a fast diagnostic, giving near immediate visual outputs, all with minimal inconvenience to the patient.
However, like serum biomarkers, several factors still stand in the way of imaging tests replacing biopsy as the gold standard NASH diagnostic. Depending on the method used, imaging can be affected by the increased fat mass as seen with in obesity (an extremely common comorbidity in those with NASH), in addition to the economic factors including adequately trained operators and the cost of any new technology needed – an important consideration in a climate where healthcare budgets continue to be squeezed.
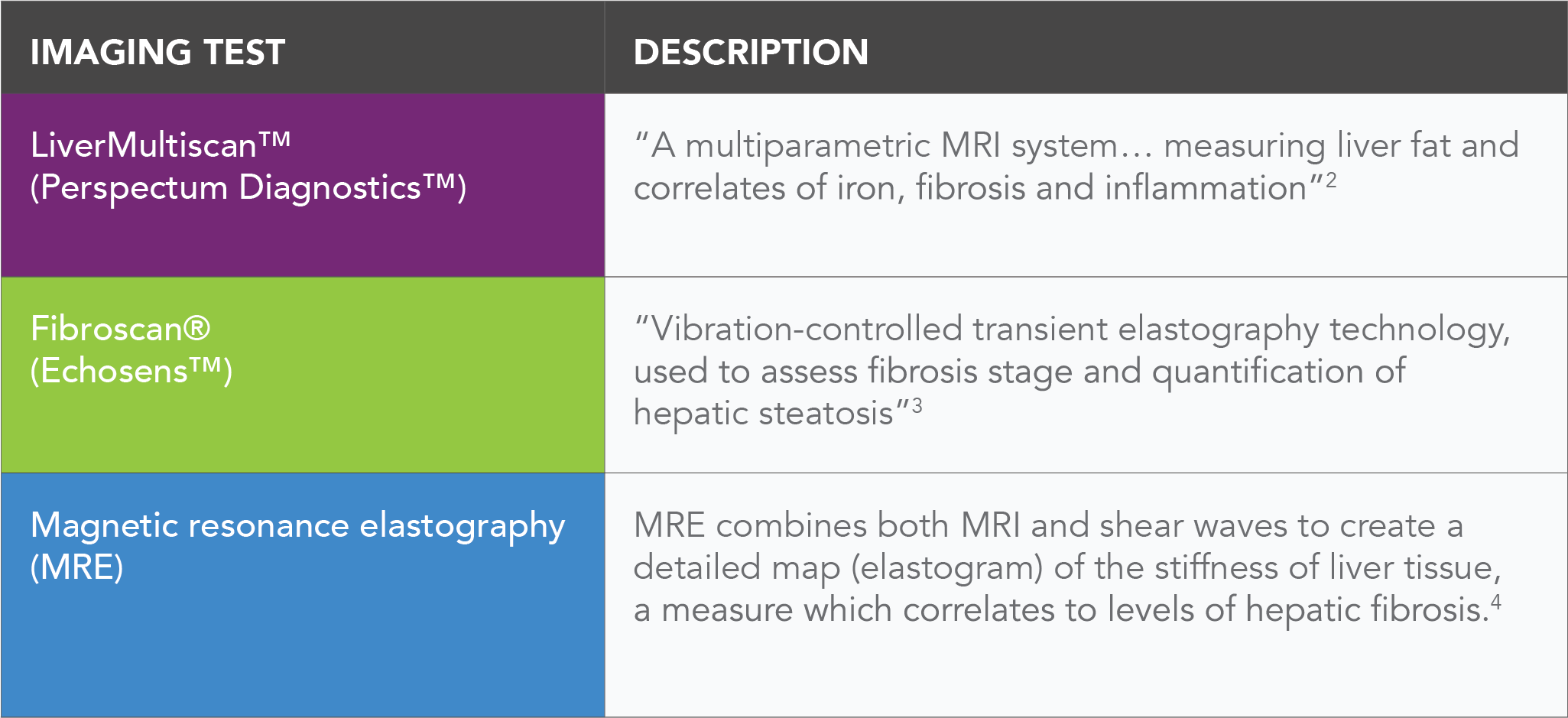
Some examples of available imaging technologies for NASH are:
Like many things in the NASH landscape, noninvasive diagnostics are still evolving and have yet to be optimized for routine care. However, as diagnosis is the key to the doorway of disease management, less-invasive diagnostic procedures could facilitate greater access to better care for the numerous people who are living unbeknown with this chronic liver disease.
For DRG’s assessment of the NASH market, please click here.
References
- Angulo P et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015 Aug;149(2):389-97.
- Perspectum Diagnostics, LiverMultiScan (https://perspectum-diagnostics.com/technology-and-services/livermultiscan; accessed 4 June 2018).
- Echosens, Fibroscan; The reference solution for non-invasive liver diagnosis (http://www.fibroscan.com/en/products; accessed 4 June 2018).
- Mayo Clinic, Patient Care & Health Information -Tests & Procedures – Magnetic resonance elastography (https://www.mayoclinic.org/tests-procedures/magnetic-resonance-elastography/about/pac-20385177; accessed 4 June 2018).





