Timely recommendation for reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. A study by the Centre for Innovation in Regulatory Science (CIRS) has demonstrated that HTA outcomes and timelines vary globally. The following article summarizes key findings from the latest CIRS R&D Briefing, Review of HTA outcomes and timelines in Australia, Canada and Europe 2016-2020.
CIRS has been benchmarking HTA agencies since 2014 using a methodology developed with agencies that ensures like-for-like comparisons. The resulting analyses, which are published annually, give unique insights into HTA processes and practices, identify where improvements can be made and inform company and agency strategies.
In this study, CIRS collected data on new active substances (NASs) appraised between 2016 and 2020 by eight HTA agencies in Canada, Australia and Europe, analyzing synchronization between the regulatory decision and the first HTA recommendation in terms of timing and outcome. Recommendations were collected from the Australian Pharmaceutical Benefits Advisory Committee (PBAC), Canadian Agency for Drugs and Technologies in Health (CADTH; both Common Drug Review [CDR] and pan-Canadian Oncology Drug Review [pCODR]), English National Institute for Health and Care Excellence (NICE), French Haute Autorité de Santé (HAS), German Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG), Polish Agencja Oceny Technologii Medycznych i Taryfikacji (AOTMiT), Scottish Medicines Consortium (SMC) and Swedish Tandvårds- & läkemedelsförmånsverket (TLV), for NASs approved 2012-2020 by the respective jurisdictional regulatory agencies, the Australian Therapeutic Goods Administration (TGA), Health Canada and European Medicines Agency (EMA).
- Number of appraisals decreased in 2020
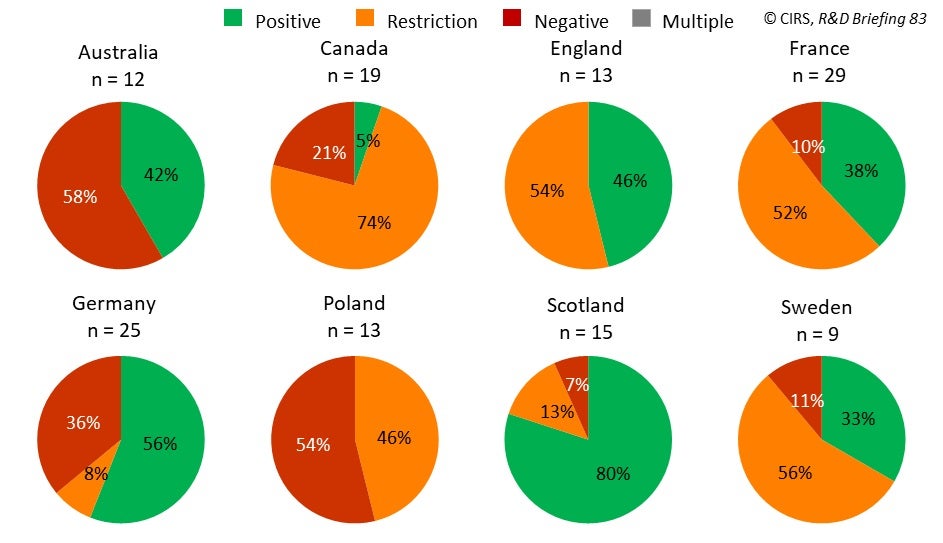
In 2020, France appraised the highest number of NASs and England had the highest proportion of positive/positive with restrictions recommendations for NASs appraised by HTA agencies (Figure 1). For all the studied countries, the number of NAS appraisals decreased in 2020 compared to the average number between 2016 to 2019, with the highest reduction occurring in England (2016-2019 average was 24 NASs vs. 2020 13 NASs). This global reduction in the number of NAS appraisals had not been observed previously between 2016 to 2019, and so may be related to the COVID-19 pandemic.
Figure 1: Comparison of first HTA recommendations across eight key jurisdictions. ‘Restriction’ refers to positive recommendations that are more restricted than the approved regulatory label and/or have conditions attached.
- Overall rollout times varied across jurisdictions
The time taken from regulatory submission to HTA recommendation (overall rollout time) includes company time dedicated to submission strategy and pre-submission preparation, as well as regulatory and HTA agency review time. In 2020, Australia had the fastest median rollout time from regulatory submission to first HTA recommendation (428 days), followed by Canada (544 days), Germany (606 days), France (678 days), England (726 days), Scotland (759 days), Poland (901 days) and Sweden (954 days). There were wide variations in rollout time across the eight jurisdictions. Germany showed the least variation in rollout time, suggesting better predictability for companies’ marketing strategies.
- Post-approval timelines were similar for expedited vs standard regulatory reviews
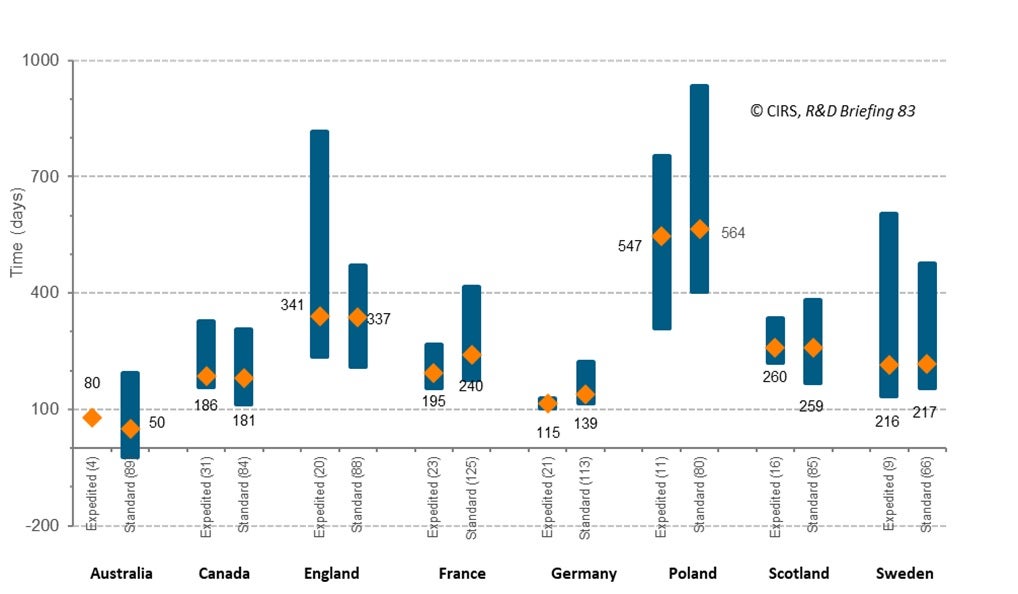
All regulatory agencies in Australia, Canada and Europe offer an expedited process designed to hasten the review process of promising NASs. This is referred to as ‘Accelerated Assessment’ by the EMA and ‘Priority Review’ by Health Canada and TGA (Priority Review was introduced by TGA in 2017). The median time from regulatory approval to HTA recommendation was similar for NASs that underwent expedited review compared to those that underwent standard review in all jurisdictions, except for France, where standard review was 45 days longer than expedited review (Figure 2).
Figure 2: Rollout time from regulatory approval to HTA recommendation (2016-2020) by regulatory review type
- Heterogeneity in HTA approaches
When considering the 26 NASs appraised by all seven HTA agencies between 2016 and 2020 (Poland was excluded due to variation), England had the highest proportion of positive or positive with restriction recommendations (100%), followed by France (96%). In comparison, Australia and Germany had the lowest percentages of positive HTA recommendations (46% and 54% of the NASs appraised, respectively). NASs were mostly likely to receive a restrictive recommendation in Canada (73% of the 26 products). In this cohort, none of the NASs had the same first HTA recommendation across all seven countries, which may reflect the heterogeneity in HTA approaches and in agency decision making.