What is the common point between ‘Aircare Tab’ and ‘Nocotine’? The answer is that they are both incrementally modified drugs (IMD) with new salt formation. Currently, Korean pharmaceutical companies mainly produce new drugs, IMDs, and generics. The new drug literally means a medicine with newly developed structure and efficacy. Generics, which are so-called “copied drugs”, are drugs that have the same function as drugs released already. Generally, when new drug patents expire, these generics are released and supplied to the public at lower prices. Finally, the last one is an IMD which will be dealt with in this article. An IMD is an improved drug formulation obtained through changes in formulas and salt changes. In the case of new drugs, the cost of research and development is high, while the IMD has the advantage of being able to be recognized as ‘innovative’ while reducing the cost of such research, and to supply safer and more effective drugs to the market. Therefore, Korean pharmaceutical companies place much emphasis on these IMDs.

But there are new obstacles to the development of the IMDs. The most important ruling came on January 17, 2017, Supreme Court 2017다245798 (Related Darts case: 2018카합21690 darts-836-918-F-en.) The issue began three years ago when Japanese pharmaceutical company Astellas filed a patent infringement lawsuit against a Korean pharmaceutical company, CorePharm Bio. Until the Supreme Court decision in 2019, the Patent Tribunal and the Patent Court found that the new salt formation does not fall within the scope of the patents sustained. This is the essence of the judgment of the Patent Court.

[The defendant’s product is the same as the patented invention and the active ingredient in this case, “Solifenacin”, and only the salt is not “succinic acid” but “fumaric acid”. In this case, there are no regulations such as: “a drug containing a new salt as an active ingredient is regarded as an item substantially the same as a drug containing another salt as an active ingredient, so that a manufacturing and importing item can be obtained,” or “the drug with new salt formations is substantially the same as a drug that has been approved as an imported item, so that there is no need to obtain a separate license for the manufacture and import of medicines “. In the end, the defendant’s product, which is based on ‘Solifenacin fumarate’, is different from the drug based on ‘Solifenacin succinate’, and it must be approved for manufacture and sale separately from drugs containing ‘solifenacin succinate’ as its main ingredient. Accordingly, the patent right of the patented invention in this case, in which the duration of the patent has been extended due to the permission of the imported product for the medicine containing ‘solifenacin succinate as its main ingredient’, is not effective to the defendant’s product which is not related to the conduct of the patented invention relating to the object.]
In accordance with the ruling of the Patent Court and the Patent Court, domestic pharmaceutical companies have actively pursued the development of salt-changed IMD and its domestic marketing. However, in January this year, the Supreme Court overturned the existing ruling. The Supreme Court’s ruling is as follows.
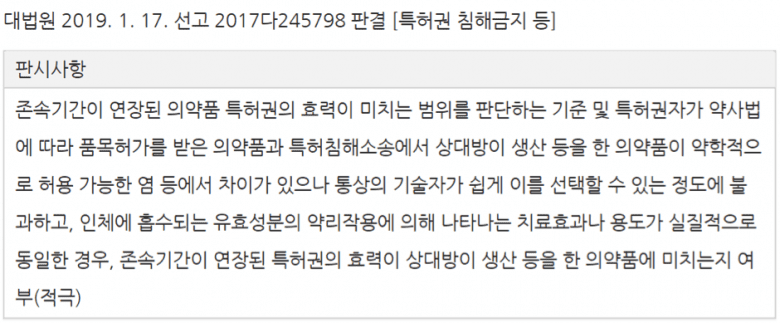
[If there are differences between the patented drug product that is licensed under the Pharmaceutical Affairs Law and the pharmacologically acceptable salt of the drug that the counterpart produces in the patent infringement lawsuit, but it is only enough for ordinary technicians to choose easily, and the therapeutic effect or use indicated by the pharmacological action of the active ingredient being administered is substantially the same, the extended patent term applies to the counterpart’s drug product.]
That is, the salt-altered IMD is also included in the validity of the extended patents. As the ruling of the Supreme Court, which is contrary to the existing ruling, was passed, domestic pharmaceutical companies are under emergency. Due to the nature of Korean pharmaceutical companies focusing on the development of generics and IMDs, there are more than 130 IMD product lists. In addition, domestic drug makers are expected to lose out in over 170 cases of IMD salt-altered patent avoidance litigation. In addition, foreign pharmaceutical companies are expected to continue to apply for injunctive relief and damages lawsuits. In fact, domestic pharmaceutical companies that have released salt replacement drugs after the Supreme Court ruling often stopped selling products to avoid such damage lawsuits.
Domestic pharmaceutical companies have also filed lawsuits against these Supreme Court rulings. Hanmi Pharmaceutical filed ‘Champix’ patent invalidation trial and extended duration invalid judgment against Pfizer. Furthermore, Hanmi Pharmaceutical and seven other pharmaceutical companies have filed a defensive confirmation trial for the scope of a right. ‘Champix’ was developed by Pfizer as a supplemental treatment of smoking cessation, and Hanmi Pharm succeeded in developing IMD(‘Nocotine’) and earned a lot of profits. If Hanmi succeeds in the aforementioned lawsuits, it will attract attention as a breakthrough in the development and sales of IMDs by domestic pharmaceutical companies. Lawsuits regarding Pfizer’s anti-smoking supplemental treatment ‘Champix’ and Boehringer Ingelheim’s anticoagulant ‘Pradaxa’ are scheduled to be sentenced by the Patent Court at the end of May.
The domestic pharmaceutical industry claims that the Supreme Court’s ruling on salt changed IMDs protects the rights of overseas pharmaceutical companies more than domestic pharmaceutical companies, and if the ruling is overextended, it will hurt the competitiveness and growth of domestic pharmaceutical companies. If so, what decisions will be made about these IMDs in overseas countries?
First, Korea and Japan are adopting ‘main component theory’. This is a view that if you use the same ingredient as the main ingredient in the new drug, it is an infringement while if you only change ‘salt formation’, it is not. Therefore, the patent tribunal and the Patent Court used to have raised the hands of domestic pharmaceutical companies by noting that the salt modified IMD is not a patent infringement. In contrast, the United States and Europe are taking “active ingredients” theory. This theory does not refer to “salt change” as a patent infringement criterion, but to “active ingredient” such as “Solifenacin” as its standard, and if the active ingredient is contained, it infringes the patent of the original drug. The recent Supreme Court case, like the United States and Europe, seems to have taken on an active ingredient theory, which has confused the domestic pharmaceutical industry.
Considering the patent rights of original drugs, the trust of domestic pharmaceutical companies, and the interests of consumers who spend drugs, it is worth closely following how patent courts will decide.





