The growing trend of physicians dispensing of oral oncolytics directly to patients disrupts the U.S. reimbursement system’s traditional reliance upon insurers’ specialty pharmacies.
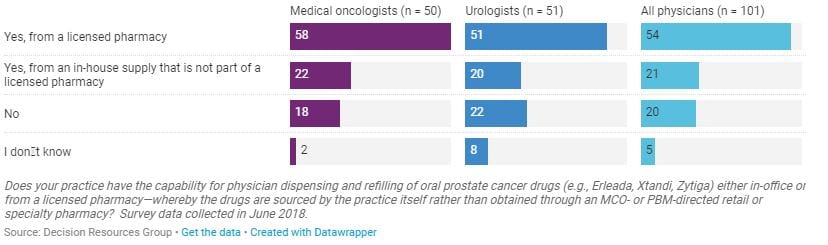
Key finding: Three quarters of oncologists and urologists surveyed by DRG reported that their practices have the capability to dispense oral prostate cancer drugs through their practice’s licensed pharmacy (most common) or from an in-house supply. This trend, often called in-office dispensing (IOD), enables patients to get their oral drugs directly from their physician’s office or on-site pharmacies, instead of using their insurers’ specialty pharmacies. The practice can save time for the patient but also provides an extra revenue stream for the practice.
The “so what” for decision makers: Oncologists have historically administered IV cancer drugs to their patients and sought reimbursement from insurers. Increasingly, as more oral therapies come to market, they are increasingly dispensing oral products, such as Xtandi and Zytiga, from provider-owned pharmacies or in-office stock. Although some managed care organizations are concerned the practice diverts valuable scripts away from their specialty pharmacies, many do accommodate the practice. This report will help drug developers with oral products understand the dynamic reimbursement and purchasing system resulting from IOD and impact on prostate cancer therapies.
Learn more about the related DRG report:
Key questions answered in the full report:
- How frequently do oncologists and urologists dispense Janssen’s Erleada and Zytiga and Pfizer’s Xtandi from their own dispensaries or pharmacies?
- What advantages do physicians report from in-office dispensing?
- How do MCOs view and react to in-office dispensing and how does this affect physician reimbursement?
- What is the coverage and prescribing environment for metastatic castrate-resistant prostate cancer therapies in general, including IV agents Provenge, Xofigo and Jevtana?
- What is the market access outlook for emerging therapies for nonmetastatic castrate-resistant prostate cancer, including Erleada and Xtandi, as well as Bayer Healthcare/Orion’s darolutamide?
Therapies studied: Erleada, Jevtana, Provenge, Xofigo, Xtandi, Zytiga, darolutamide
Companies mentioned: Johnson & Johnson (Janssen), Pfizer, Astellas, Bayer Healthcare, Sanofi, Sanpower Group/Dendreon





